* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Blood Coagulation Pathway
Drug design wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Pharmacognosy wikipedia , lookup
Prescription costs wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Drug discovery wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Theralizumab wikipedia , lookup
Metalloprotease inhibitor wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Neuropharmacology wikipedia , lookup
Drug interaction wikipedia , lookup
Dydrogesterone wikipedia , lookup
Discovery and development of direct Xa inhibitors wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
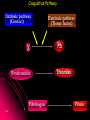
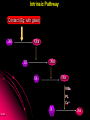
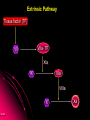
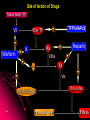
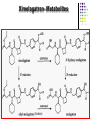
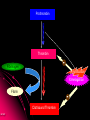
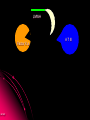
ORAL DIRECT THROMBIN INHIBITORS Girish Girish C & M Jayanthi Dept of Pharmacology JIPMER Coagulation Pathway Intrinsic pathway (Contact) X Prothrombin Fibrinogen Girish Extrinsic pathway (Tissue factor) Xa Xa Thrombin Thrombin Fibrin Intrinsic Pathway Contact (Eg: with glass) XII XIIa XIa XI IXa IX VIIIa PL Ca++ Girish X Xa Extrinsic Pathway Tissue factor (TF) VIIa- TF VII XIa IX IXa VIIIa X Girish Xa Site of Action of Drugs Tissue factor (TF) VII VIIa- TF - IXa VIIIa IX - Warfarin - - Heparin Xa X - Va Thrombin Prothrombin Girish TFPI,NAPc2 Fibrinogen Fibrin Why new oral anticoagulants ? Warfarin- sole oral anticoagulant for 60 years. Limitations- Narrow therapeutic index Delayed onset & offset of action Mandatory lab monitoring Drug interactions Girish Warfarin Therapy Clotting Bleeding Features of an ideal anticoagulant High efficacy to safety index Predictable dose response Administration by parenteral and oral routes Rapid onset of action Availability of a safe antidote Freedom from side effects Minimal interactions Girish Why thrombin is an excellent target? Forms & stabilizes the clot Further generation of thrombin Stimulates thrombus- activated fibrinolysis inhibitor (TAFI) resulting in inhibition of fibrinolysis Activates platelets Girish Direct Thrombin Inhibitors (DTIs) Directly binds and inhibit thrombin Action not mediated through antithrombin Hirudin from Hirudo medicinalis Lepirudin – Approved for thrombosis related to Heparin Induced Thrombocytopenia (HIT) Bivalirudin – For percutaneous coronary angioplasty Girish Parenteral DTIs: Bivalent: Monovalent: Hirudin Argatroban Bivalirudin Melagatran Lepirudin Oral DTIs: Ximelagatran Girish Ximelagatran Competitive and reversible direct thrombin inhibitor. Inhibits thrombin activity, thrombin generation and platelet activation. Prolongs aPTT, Prothrombin time. No action on other serine proteases Girish Pharmacokinetics Girish Ximelagatran- Metabolites (Active) Kinetics… Prodrug of melagatran About 20% of oral dose is absorbed. Tmax - 2-3 hrs, t1/2 – 4-5 hrs Eliminated via kidney (80%) Kinetics not influenced by sex, body weight and ethnicity Girish Pharmacodynamics Girish Prothrombin Thrombin - Fibrinogen Amplification Ximelagatran Fibrin Clot bound Thrombin Girish Exosite 2 Thrombin Active site Melagatran Exosite 1 Fibrinogen recognition site Girish Thrombin Hirudin Girish Thrombin Bivalirudin Thrombin Girish Thrombin Argatroban Thrombin Girish Heparin Thrombin AT III Girish LMWH Factor X a Girish A T III Non- hemorrhagic adverse effects: Elevation in alanine transferase values in 6-10% patients Developed with in 6 wks to 6 months Normalized after discontinuation Purpura, dizziness, edema, diarrhea, fatigue Girish Drug Interactions Girish Interactions… Metabolism independent of CYP 450 No binding to plasma proteins or platelets No drug- food interaction Less propensity for drug interactions. Girish Advantages Administered orally at Fixed doses Coagulation monitoring not required Immediate action More predictable anticoagulant response Wider safety margin Less inter subject variability Minimal drug interactions Girish Disadvantages: Hepatotoxicity No antidote available (but dialysis can hasten elimination) Girish Indications & Dose Long term secondary prevention of VTE after standard therapy for an episode of acute VTE (24mg bid) Post operatively for the prevention of VTE after TKR surgery ( 36 mg bid) Prevention of stroke in atrial fibrillation (36mg bid) Girish Clinical trials Girish SPORTIF III& IV: Ximelagatran not inferior to warfarin for preventing stroke with no difference in bleeding rates. METHRO III & EXPRESS: Ximelagatran is as effective as standard therapy for VTE after major orthopaedic surgery. THRIVE II& V: Ximelagatran is as efficacious as standard Girish therapy for treating DVT with similar bleeding incidents Current Status NDA submitted on Dec 23, 2003 US FDA approval pending In Europe, approved for short term orthopaedic prophylaxis. Girish Conclusion Girish Conclusion… Favorable pharmacokinetic & dynamic profile Efficacy similar to warfarin No data in pregnancy, lactation and children Hepatotoxicity Benefit- risk considerations Girish Will ximelagatran replace warfarin ? Has many features of an ideal anticoagulant Hepatotoxicity is of concern Newer oral DTIs are being developed (Dabigatran etexilate) If these are devoid of hepatotoxicity, it can replace warfarin Girish Girish Reviews: 1. Hirsh J, O’Donnell M, Weitz JI. New anticoagulants. Blood,2005;105:453-63. 2. Boos CJ, More RS. Anticoagulation for non-valvular atrial aibrillation – towards a new beginning with ximelagatran. Curr Control Trials Cardiovasc Med. 2004;5:3-10. 3. BrightonTA. The direct thrombin inhibitor melagatran/ximelagatran, Med J Aust 2004; 181: 432–37. 4. Kokolis S, Cavusoglu E, Clark LT, Marmur JD. Anticoagulation strategies for patients undergoing percutaneous coronary intervention: Unfractioned heparin, low- molecular- weight heparins, and direct thrombin inhibitors. Prog Cardvasc disease 2004;46: 506-23. Girish Original Papers: 1. Clement B, Lopian K. Chacterization of in vitro biotransformation of new, orally active, direct thrombin inhibitor ximelagatran, an amidoxime and ester prodrug, Drug Metab Dispos 2003;31:645–51. 2. Eriksson UG, Johansson S, Attman PO, Mulec H, Frison L, Fager G, Samuelsson O. Influence of severe renal impairment on the pharmacokinetics and pharmacodynamics of oral ximelagatran and subcutaneous melagatran. Clin Pharmacokinet. 2003;42:743-53. 3. Schulman S, Wahlander K, Lundstrom T, Clason AB, Eriksson H. Secondary prevention of venous thromboembolism with the oral direct thrombin inhibitor ximelagatran. N Engl J Med 2003;349:1713-21. 4. Bredberg E, Andersson TB, Frison L, Thuresson A, Johansson S, Eriksson-Lepkowska M, Larsson M, Eriksson UG. Ximelagatran, an oral direct thrombin inhibitor, has a low potential for cytochrome P450-mediated drug-drug interactions. Clin Pharmacokinet. 2003;42:765-77. Girish 5. Albers GW, Diener HC, Frison L, Grind M, Nevinson M, partridge S, et al. SPORTIF Executive Steering Committee for the SPORTIF V Investigators. Ximelagatran vs warfarin for stroke prevention in patients with nonvalvular atrial fibrillation: a randomized trial. JAMA. 2005 ;293:690-8. 6. Fiessinger JN, Huisman MV, Davidson BL, Bounameaux H, Francis CW, Eriksson H, et al. THRIVE Treatment Study Investigators. Ximelagatran vs low-molecular-weight heparin and warfarin for the treatment of deep vein thrombosis: a randomized trial, JAMA.2005 ;293(6):681-9. Girish Editorials: 1. Gurewich V. Ximelagatran—Promises and Concerns, JAMA 2005; 293:736-9. 2. Shapiro S S. Treating thrombosis in the 21st century. N Engl J Med 2003;18: 1762-4. Girish Thank You Girish