* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Electric Cells - Physics Rocks!
Voltage optimisation wikipedia , lookup
Opto-isolator wikipedia , lookup
Stray voltage wikipedia , lookup
Buck converter wikipedia , lookup
Current source wikipedia , lookup
Distribution management system wikipedia , lookup
Mains electricity wikipedia , lookup
Alternating current wikipedia , lookup
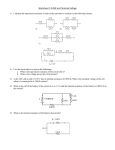
Electric Cells Section 5.3 (p. 217 – 226) (chemical) Cells • • • • • i.e. batteries Direct current (DC) devices Electron flow = leave negative side, re-enter positive terminal Conventional current = positive terminal negative terminal Higher potential = positive terminal • Primary Cell • Internal cell • Used until power supply is exhausted • Secondary Cell • Rechargeable • When exhausted, charger reverses chemical reaction to recreate original chemical composition Chemistry connection: • What are the chemicals used in a primary cell dry-cell or battery? • Which is associated with the positive terminal and which is associated with the negative terminal? • What is different about a rechargeable battery? • Email me your answers, with citation of your source(s), by Wednesday morning (9:55 AM), for up to 3 assignment e.c. points Cell Capacity • If two cells have the same chemical composition, they will each be able to generate the same emf (electromotive force) for a circuit. • Capacity: the measure of the ability of a cell to release its charge. • High rate of discharge = short-lasting cell • Low rate of discharge = long-lasting cell • Determined by the constant current that can be supplied during discharge • Units = Amp-hours (Ah) Lab set-up • Select someone in the class to set up a scenario such as is found on page 221 of your textbook. Use a LabQuest, a 1.5 V battery, a light-bulb, and a Vernier Voltage Sensor. • We will be leaving this set up and collecting data overnight. (set the data collection accordingly—for about 30 hours, with data collected every minute or so) • If you are not the one setting it up, you need to check on it. • Journal entry for everyone: Sketch a circuit diagram of the set-up, and predict what the graph of terminal voltage as a function of time will look like for the next 26 hours. Leave space for a sketch of the actual results. eMF • Electromotive Force: • the open circuit potential difference across the terminals of a power source • The terminal voltage when no current is supplied • The energy per unit charge made available (supplied) by the source • Terminal Voltage: • The potential difference measured across the terminals of a cell or battery Internal Resistance • How would internal resistance affect the emf of a cell? • How would internal resistance affect the terminal voltage of a cell? • Internal Resistance • Present in all electrical cells, to some extent • Consumes some of the potential from the eMF, so the terminal voltage will decrease when current is running through a circuit. • eMF = terminal voltage + potential drop across internal resistance 𝜀 = 𝑉 + 𝐼𝑟 or 𝜀 = 𝐼(𝑅 + 𝑟) Packet q #6 • In the circuit below an electrical device (load) is connected in series with a cell of emf 2.5 V and internal resistance r. The current in the circuit is 0.10 A. e.m.f. = 2.5V • The power dissipated in the load is 0.23 W • Calculate: r • The total power of the cell I = 0.10A • Resistance of the load load • Internal resistance of the cell Q6, continued • A second identical cell is connected into the circuit as shown below I = 0.15A load • The current in this circuit is 0.15 A. Deduce that the load is a non-ohmic device.