* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The Wave
Atomic orbital wikipedia , lookup
Tight binding wikipedia , lookup
Delayed choice quantum eraser wikipedia , lookup
Bremsstrahlung wikipedia , lookup
Renormalization wikipedia , lookup
Electron configuration wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Elementary particle wikipedia , lookup
Wheeler's delayed choice experiment wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Particle in a box wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
Atomic theory wikipedia , lookup
Double-slit experiment wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Matter wave wikipedia , lookup
Wave–particle duality wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
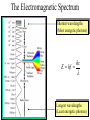
The Wave – Particle Duality OR Light Waves Until about 1900, the classical wave theory of light described most observed phenomenon. Light waves: Characterized by: Amplitude (A) Frequency (n) Wavelength (l) Energy a A2 And then there was a problem… However, in the early 20th century, several effects were observed which could not be understood using the wave theory of light. So other experiments were done and found it could behave as both:: 1) The Photo-Electric Effect (particle) 2) The Compton Effect (particle) 3) Taylor’s experiment (wave) 4) DeBroglie Wavelength (wave) Quantized Model of Light (Photons) • In 1900 Max Planck proposed that light energy comes in packets (quanta) spread at random on a wave front called PHOTONS. • He even doubted his idea since: It went against wave theory by saying that electromagnetic waves don't transmit energy continuously but in small packets. It went against Newtonian physics since objects aren't free to vibrate with any energy. The energy only has certain discreet values. Quantized Model of Light Energy of a light particle (Photon): E photon hf hc l h = 6.6x10-34 [J*sec] Planck’s constant, after the scientist Max Planck The Electromagnetic Spectrum Shortest wavelengths (Most energetic photons) E hf hc l Longest wavelengths (Least energetic photons) Photons Problems: 1. Find the energy in electron volts (eV) for a quantum of orange light with frequency 5.00 x 1014 Hz. (Answer: 2.07eV) 2. Find the energy in electron volts for a quantum of yellow light with a wavelength of 580 nm. (Answer: 2.14 eV) Suggested Textbook Problems: Page 597 #2-6 Photoelectric Effect “Classical” Method What if we try this ? Increase energy by increasing amplitude Vary wavelength, fixed amplitude electrons emitted ? No No No No electrons emitted ? No Yes, with low KE Yes, with high KE No electrons were emitted until the frequency of the light exceeded a threshold frequency, f o at which point electrons were emitted! Photoelectric Electrons are attracted to the (positively charged) nucleus by the electrical force In metals, the outermost electrons are not tightly bound If given energy electrons can be freed Classically, we increase the energy of an EM wave by increasing the intensity (e.g. brightness) Energy a A2 But this doesn’t work ?? PhotoElectric Effect An alternate view is that light is acting like a particle The light particle (photon) must have enough energy to “free” the electron Increasing the Amplitude is just simply increasing the number of light particles, but its NOT increasing the energy of each one! However, if the energy of these “light particle” is related to their frequency, this would explain why higher frequency light can knock the electrons out of their atoms, but low frequency light cannot… Photo-Electric Effect • See diagram below …the energy of the light particle (photon) must overcome the binding energy of the electron to the nucleus (Work) • Ephoton = Ek + Work. • Ek = Ephoton – Work. (Einstein’s Photoelectric Equation) “Light particle” Metal Surface “Freed” electron has Ek Work to free elctron Photo-Electric Problems 1. EM radiation of frequency 7.0 X 1014 Hz falls on a metal with work function of 0.5eV. a) Calculate the maximum kinetic energy of the emitted photoelectrons and the maximum speed of the emitted photoelectrons. (Answer: 3.8 x 10 –19 J) NOTE: Remind students if questions ask for speed make sure you convert everything into Joules. b) What would be the case if the kinetic energy was less than 0.5 eV. (Answer: no emission) Photo-Electric Problems 2. Calculate the Threshold (minimum) frequency for a metal with a work function or binding energy of 1.5 eV. (Answer: 3.6 x 1014) 3. A photoelectric surface has a work function of 1.50 eV. A red light of wavelength 650 nm is directed at the surface. Calculate: a) The maximum Ek of the emitted photoelectrons in joules (Answer: 6.60 x 10 -20 J) b) The photoelectrons' maximum speed (Answer: 3.81 x 10 5 m/s) c) The cutoff potential needed to stop the photoelectrons (Answer: 0.412 V) Photo-Electric Problems Suggested Text Questions: Pg. 604 #10-15 The Compton Effect 1924 Compton performed the photoelectric exp with Xrays . Like the photoelectric effect it showed light behaving as a particle. Incident X-ray l1 M A T T E R Scattered X-ray l2 e Electron comes flying out The Compton Effect Energy and momentum are conserved Exray photon ' E xray photon Ek electron Notice: There is NO work function since it is negligible (compared to x-ray energy). The Compton Effect Compton derived the expression for momentum of a photon. E mc 2 p h l E photon mc 2 mc E photon p photon p photon c hf c h l Bohr’s Complementarity Principle If light is passing through a medium treat it like a wave. If light is reacting with matter treat it as a particle. Wave Nature of Matter DeBroglie thought: “If light waves can behave like a particle, might particles act like waves”? DeBroglie’s wavelength: h h l p mv Matter Waves (cont) Ex 1: Compute the wavelength of a 1 [kg] block moving at 1000 [m/s]. l = h/mv = 6.6x10-34 J s/(1kg)(1000 m/s) = 6.6x10-37 [m]. VERY small. therefore wave behavior of matter can’t be seen. Electron Microscope The electron microscope is a device which uses the wave behavior of electrons to make images which are otherwise too small using visible light! This image was taken with a Scanning Electron Microscope (SEM). These devices can resolve features down to about 1 [nm]. This is about 100 times better than can be done with visible light microscopes! IMPORTANT POINT HERE: High energy particles can be used to reveal the structure of matter ! High Energy Particles High energy particles can provide a way to reveal the structure of matter beyond what can be seen using an optical microscope. The higher the momentum of the particle, the smaller the deBroglie wavelength. As wavelength decreases, finer and finer details about the structure of matter are revealed ! This is done at facilities often referred to as “atomsmashers” or “accelerators”