* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Central Hypothyroidism - e
Survey
Document related concepts
Hormone replacement therapy (male-to-female) wikipedia , lookup
Signs and symptoms of Graves' disease wikipedia , lookup
Hypothalamus wikipedia , lookup
Growth hormone therapy wikipedia , lookup
Pituitary apoplexy wikipedia , lookup
Hyperthyroidism wikipedia , lookup
Transcript
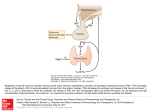
CME Review Article #16 0021-972X/2002/1203-0218 The Endocrinologist Copyright © 2002 by Lippincott Williams & Wilkins CHIEF EDITOR’S NOTE: This article is the 16th of 36 that will be published in 2002 for which a total of up to 36 Category 1 CME credits can be earned. Instructions for how credits can be earned appear after the Table of Contents. Central Hypothyroidism A. Gudmundsdottir, M.D.* and J. A. Schlechte, M.D.† missed if a TSH is the only screening test used for detection of thyroid disease, and we recommend measurement of both free thyroxine and TSH for screening purposes. The treatment is the same as in primary hypothyroidism except that TSH cannot be used to monitor therapy. It is important to assess the pituitary adrenal axis before starting levothyroxine to avoid precipitation of adrenal crisis in patients with adrenal insufficiency. ■ Most patients with hypothyroidism have thyroid failure or primary hypothyroidism. Central hypothyroidism (CH) is caused by impaired stimulation of a normal thyroid gland by hypothalamic or pituitary hormones. Most cases of CH are caused by pituitary tumors, but genetic defects causing abnormalities in the thyroid-stimulating hormone (TSH) subunit also lead to CH. The symptoms are similar to those of primary hypothyroidism, and most patients with CH will also have signs and symptoms of other anterior pituitary dysfunction. The diagnosis of CH can be The Endocrinologist 2002; 12: 218–223 roid failure that occurs as a result of TSH or TRH deficiency. Some authors prefer the terms secondary (TSH deficiency) and tertiary (TRH deficiency) hypothyroidism based on the response of TSH after administration of TRH. However, there is frequent overlap between secondary and tertiary forms, and CH is a more appropriate term [1]. Learning Objectives • Identify the causes and pathogenetic mechanisms underlying acquired, congenital, and transient central hypothyroidism (CH). • Describe the clinical and endocrinological characteristics of CH. • Recall the most effective treatment of CH, the best marker of treatment response, and the perils of undertreatment and overtreatment. Pathogenesis Thyrotropin-releasing hormone is a 26-kd protein that occurs in the hypothalamus, pituitary, gastrointestinal tract, pancreatic islets, and reproductive tract. It has a halflife of 3 minutes and stimulates the secretion, synthesis, and glycosylation of TSH. In healthy subjects, the administration of TRH causes a dose-dependent increase in TSH, prolactin, and growth hormone (GH). Thyroid-stimulating hormone is synthesized and secreted by the thyrotrophs of the anterior pituitary. It is a 28kd glycoprotein composed of and subunits and contains approximately 15% carbohydrate. The subunit is unique and determines the biologic specificity. The unit is identical to the subunit of luteinizing hormone, folliclestimulating hormone, and chorionic gonadotropin. Thyroidstimulating hormone secretion is pulsatile, peaking in the late evening. Variation in the carbohydrate chains has a major impact on the biological properties of TSH. Highly sialylated TSH has impaired intrinsic bioactivity and a long Introduction P ❦ ituitary secretion of thyroid-stimulating hormone (TSH) occurs in response to release of the hypothalamic-releasing hormone, thyrotropinreleasing hormone (TRH). Central hypothyroidism (CH) is the term used to describe thy- *Fellow in Endocrinology and Metabolism and †Professor of Medicine, University of Iowa Hospitals and Clinics, Iowa City, Iowa, U.S.A. Address correspondence to: J. A. Schlechte, M.D., Department of Internal Medicine, University of Iowa Hospitals and Clinics, 200 Hawkins Drive, Iowa City, IA 52242. Telephone: 319-384-8305; Fax: 319-384-8325; E-mail: [email protected]’ The authors have disclosed that they have no significant relationships with or financial interests in any commercial company pertaining to this educational activity. 218 Central Hypothyroidism half-life. In normal circumstances, isoforms of TSH secreted at night have a higher degree of sialylation than isoforms secreted during the day, accounting for a lower bioactivity ratio [2]. In adults with primary hypothyroidism, TSH isoforms are also highly sialylated with impaired bioactivity [2]. On the contrary, a normal fetus has extremely bioactive asialoTSH isoforms circulating during the third trimester. Hypothalamic TRH exerts its effects by regulating TSH posttranslational oligosaccharide processing and release of TSH [2]. This results in multiple molecular forms of circulating TSH in variable physiological and pathological states. Subjects with primary or CH have abnormalities in pulsatile TSH secretion, including a blunted or absent nocturnal surge. Thyroid-stimulating hormone is secreted into the systemic circulation and stimulates growth of the thyroid gland, along with synthesis and release of thyroid hormones. Thyroid hormones then exert negative feedback at the hypothalamus and pituitary to regulate TRH and TSH levels [3]. Etiology Acquired Central Hypothyroidism In most cases, CH develops in patients with pituitary and/or hypothalamic diseases. This may be caused by a reduced mass of functioning thyrotrophs, defects in TRH stimulation, or a reduced bioactivity of circulating TSH [4]. Table 1 lists a variety of causes of CH. Pituitary adenomas are the most common cause of CH [3]. A tumor may produce other pituitary hormones or lead to decreased TSH production from compression of the gland. Meningiomas and craniopharyngiomas can cause CH, but metastatic tumors to the hypothalamus and pituitary are a rare cause. In patients with postpartum pituitary necrosis (Sheehan syndrome) and panhypopituitarism, TSH can be unexpectedly normal or increased. Circulating TSH in these patients has a higher degree of sialylation and decreased biological activity compared with that of normal subjects [5]. External radiation therapy caused CH in 65% of patients treated for brain tumors [6]. In pediatric cancer survivors who received head irradiation, subtle CH developed in 30%. Five of 62 patients had abnormal basal serum TSH or free thyroxine (FT4) levels. The other 57 had normal TSH and FT4 but had a blunted nocturnal TSH surge or delayed TSH response to TRH [7]. Hypopituitarism can also appear after excision of a pituitary adenoma. Webb et al. [8] identified TSH deficiency in 10.5% of patients with normal preoperative pituitary function. Genetic Defects Congenital CH has been described in several families as being caused by mutations in the TSH– subunit gene. The Endocrinologist Table 1 Causes of Central Hypothyroidism Tumors Benign: pituitary adenomas, cysts, craniopharyngiomas Malignant: metastatic from lung, breast, etc. Ischemic necrosis Sheehan syndrome Shock Iatrogenic Surgery Radiation Infectious Tuberculosis Infiltrative lesions Sarcoidosis Hemochromatosis Fungal infections Toxoplasmosis Syphilis Histiocytosis Autoimmune lymphocytic hypophysitis Genetic abnormalities in TSH Aneurysm of ICA Trauma Idiopathic ICA, internal carotid artery; TSH, thyroid-stimulating hormone. These mutations introduce truncated proteins that cannot dimerize with the -subunit or, alternatively, produce a mutated TSH that is biologically inactive [9,10]. Five Japanese families with familial inherited TSH deficiency have been reported [11]. Three have a single base substitution in the same area, suggesting that they originated from the same single founder. The resulting amino acid substitution affects the molecular conformation of the -subunit, making it unable to bind with the -subunit. The mutation generated a new MaeI cleavage site that can be used for DNA diagnosis. The other two families, studied by the same investigators, are expected to have a different mutation. Other families have since been described in Brazil [12], Greece [13], and other countries [10,14–16]. The inheritance pattern is usually autosomal recessive and the families are frequently highly inbred. The patients have hypothyroidism at birth. A goiter is not present and thyroid radioiodine uptake is low. In three affected children from the Greek families, there was no significant TSH response after TRH stimulation. Their mutation was found to be different from that described by Hayashizaki et al. [11] in the Japanese families. The TSH– subunit is generally undetectable in patients with mutations that prevent the assembly of the heterodimer. However, there are other mutations in which the heterodimerization is not completely prevented and the affected patients have variable serum TSH concentrations, 219 Central Hypothyroidism depending on the measurement method used [12,14–16]. This mutant heterodimer has preserved immunoreactivity but not bioactivity, which results in a reduced ratio between biologic and immunoreactive TSH. The subunit has been found to be significantly increased in these patients [10,12]. The TSH deficiency is most often isolated, with other pituitary hormones being normal. Central hypothyroidism has also been reported with a nonsense mutation in the pituitary-specific transcription activator, POU1F1 (formerly known as Pit-1) [17]. This defect is associated with other pituitary hormone deficiencies. This clinical picture may also result from gene mutations of other transcription factors such as Prop-1 [18], Hesx1 [19,20], and Lhx3 [21]. Levels of GH, prolactin, and TSH in patients with Prop-1 mutations are, on average, slightly higher than those in patients with Pit-1 mutations; however, all levels are in the subnormal range [22]. An inactivating mutation in the TRH receptor gene has been described as a cause of CH in one patient [23]. This 9-year-old boy was referred for evaluation of short stature and had markedly delayed bone maturation as the only presenting symptom. Although his total thyroxine (T4) was low, at neonatal screening he had a normal TSH. He was found to have been a compound heterozygote, having inherited a different mutated allele from each of the parents. Both mutations resulted in a receptor with reduced or absent biological activity. In addition, there is some evidence that a group of CH patients have TRH deficiency [24]. This is difficult to document because various serum components interfere with the TRH assay. As noted, TRH has a short half-life and its origin in peripheral blood is uncertain. These patients may benefit from repetitive exogenous TRH administration. Transient Central Hypothyroidism Central hypothyroidism can be temporary in patients with severe nonthyroidal illness. This includes major surgery, trauma, chronic renal failure, depression, anorexia, and fasting. It can also be found in the elderly. Thyroid-stimulating hormone can remain low for up to 1 month after treatment of hyperthyroidism, regardless of treatment method. A similar phenomenon is observed after withdrawal of T4 therapy in patients with multinodular goiter. Other medications that inhibit TSH secretion include dopamine, glucocorticoids, and somatostatin. A careful history and evaluation of the circumstances surrounding abnormal thyroid function tests are crucial to avoid unnecessary diagnostic tests. Cases of reversible CH have been described in patients with cutaneous T-cell lymphoma treated with highdose bexarotene [25]. This retinoid X–receptor-selective 220 ligand suppressed TSH secretion in 26 of 27 patients during therapy. Serum concentrations of free thyroxine decreased, also. Nineteen patients had symptoms or signs of hypothyroidism that were not present at baseline. Among 10 patients that were studied after discontinuation of the treatment, serum TSH returned to normal in nine. Congenital Hypothyroidism The prevalence of congenital hypothyroidism is approximately 1 in 4,000 births, and newborn screening is routine in the United States and other countries [26]. Because of screening, congenital hypothyroidism is no longer a significant cause of mental retardation in industrialized societies. Samples of T4 or TSH are obtained 24 to 48 hours after birth. The cause of congenital hypothyroidism includes thyroid dysgenesis (aplasia, hypoplasia, ectopy), thyroid dyshormonogenesis, transient hypothyroidism (usually caused by iodine, drugs, or maternal antibodies), and hypothalamic– pituitary hormone deficiency (also known as CH). Central hypothyroidism accounts for only approximately 5% of cases. The most common cause is thyroid malformations. Infants with abnormal test results should be recalled quickly to confirm the diagnosis and start treatment. Most infants with low FT4 (20–25 mU/L) and low TSH (20–25 U/mL) levels are premature, manifesting transient hypothyroxinemia of prematurity. If TSH deficiency is suspected, measurements of GH and cortisol may indicate panhypopituitarism. The presence of hypoglycemia in a term neonate should suggest GH and/or adrenocorticotrophic deficiency. Further evaluation should include a TRH test and imaging of the brain to identify hypothalamic–pituitary anomalies. In addition, DNA tests permit rapid identification of point mutations in the TSH– gene as discussed. Therapy in infants with CH is similar to therapy for other congenital hypothyroid states. It is extremely important to rapidly normalize the serum T4 concentration. The serum T4 should then be maintained at more than 103 nmol/L (8 g/dL) throughout the first year of treatment. Alternatively, FT4 measurements can be used and should be maintained in the upper half of the normal range for the method [26]. The recommended initial dose of T4 is 10 to 15 g/kg per day or 50 g daily for the average term infant weighing 3 to 4.5 kg. Therapy should be monitored at 4- to 6-week intervals during the first 6 months, at 2- to 3-month intervals between ages 6 and 24 months, and at 3to 6-month intervals thereafter. If the initial diagnosis cannot be established definitively, levothyroxine can be withdrawn for 30 days at age 2 to 3 years without compromising brain maturation to allow reassessment [26]. Volume 12, Number 3 Central Hypothyroidism Clinical Features The clinical features of CH are similar to those of primary hypothyroidism but generally milder. The skin may not be as coarse and dry as in primary hypothyroidism, periorbital and peripheral edema are uncommon, and hoarseness is not prominent in patients with CH [1]. Common symptoms include cold intolerance, constipation, fatigue, lethargy, muscle cramps, and weight gain. Physical findings include bradycardia, hypothermia, slow speech, and a prolonged relaxation phase of the deep tendon reflexes. Children may present with stunted growth and delay in bone development. Dwarfism and cretinism may occur in the rare familial forms. In patients with hypopituitarism, deficiency of GH and gonadotropin may precede TSH insufficiency. Delayed skeletal maturation in children may be the sign of GH deficiency. Gonadotropin insufficiency causes impotence, loss of libido, diminished beard growth, and testicular atrophy in men. Women may present with amenorrhea, infertility, and breast atrophy. Hypoglycemia may be the result of hypocortisolism, and adrenal insufficiency may result in anorexia and weight loss. Diabetes insipidus is frequently seen with craniopharyngiomas and with infiltrative diseases of the hypothalamus. Diagnosis The diagnosis of CH is based on the demonstration of low-serum thyroid hormone along with inappropriately reduced, normal, or slightly increased TSH. If serum TSH is used as the initial screening test for thyroid disease, the diagnosis of CH can easily be missed. For this reason, we agree with those who recommend measurement of both FT4 and TSH for screening. Alternatively, serum FT4 can be assessed if the patient has symptoms suggestive of hypothyroidism or hypopituitarism in the presence of a normal serum TSH. It is important to remember that most patients with CH will also have deficiencies in other pituitary hormones. Therefore, the clinical picture will vary widely. The reason for normal or high TSH levels in some patients with CH is that the TSH is bioinactive but remains immunoactive and thus measurable by immunometric assay. This finding may be partly explained by degree of sialylation of the TSH molecules in CH, which is controlled by TRH. A recent study showed that patients with hypothalamic–pituitary disease had TSH with reduced bioactivity, along with a decreased number of functioning thyrotrope cells [27]. In normal circumstances, TSH is secreted in a diurnal rhythm with a surge that begins in the late evening and reaches a peak at the onset of sleep. This nocturnal surge is absent in many patients with CH. To assess the The Endocrinologist nocturnal surge, TSH samples should be obtained every 30 minutes from 11:00 PM to 2:00 AM. A TRH stimulation test involves administration of 200 to 500 g or 5 g/kg of TRH, then measurement of TSH at 20- and 60-minute intervals after injection. A delayed response is defined as a peak serum TSH concentration that occurs 60 or more minutes after TRH administration, whereas a normal response peaks at 20 to 30 minutes [1]. Classically, this test was used to identify the cause of CH. The serum TSH response to TRH should be impaired in pituitary hypothyroidism but preserved in hypothalamic hypothyroidism. It has been shown, however, that serum TSH responses to TRH differ little in patients with hypothalamic or pituitary disorders [1]. A delayed TSH response to TRH is a characteristic but not specific finding in hypothalamic disease, and some patients with unequivocal hypothalamic disorder have a low or normal serum TSH response to TRH. Yamakita [28] studied six patients with an idiopathic isolated deficit of TSH secretion and found blunted responses to TRH stimulation in all patients without a TSH surge. They suggested that both of these tests should be performed when CH is suspected, because routine biochemical data and clinical symptoms cannot easily detect this disease. Magnetic resonance imaging of the hypothalamic area and pituitary gland is indicated in patients with biochemical evidence of CH. Computed tomography with coronal views through the pituitary is an alternative if magnetic resonance imaging is unavailable. Therapy The goal of therapy in CH is to restore and maintain euthyroidism. This is best performed with levothyroxine. Unlike primary hypothyroidism, serum TSH cannot be used for monitoring treatment in CH. Ferreti et al. [4] examined a variety of clinical and biochemical parameters as indices of thyroid hormone action in 37 patients with CH, with and without administration of levothyroxine therapy. The biochemical markers included thyroid hormone, TSH, cholesterol, sex hormone-binding protein, angiotensin-converting enzyme, carboxyl-terminal telopeptide of type I collagen, bone glucose-lowering agent protein and serum soluble IL-2 receptors. The clinical parameters (heart rate, blood pressure, body mass index, skin findings, and edema) lacked specificity for the diagnosis or follow-up of CH patients, probably because of associated hormonal deficiencies that usually do not occur in primary hypothyroidism. The most reliable marker was serum FT4. Free triiodothyronine (FT3) remained normal in 25% of patients with low FT4. Total T3 and T4 were less reliable. Optimization of therapy is extremely important because overtreatment can con221 Central Hypothyroidism tribute to increased fracture risk, which may already be present because of concomitant gonadotroph and GH deficiency. Undertreatment with levothyroxine may increase the cardiovascular risk. FT3 appeared to be more sensitive than FT4 for detecting overtreatment. FT4 was more useful in identifying undertreated CH patients. Serum levels of creatine kinase along with FT4 and FT3 may be useful in detecting undertreatment. Soluble IL-2 receptor is a sensitive marker of biological effects of thyroid hormone on lymphocytes in various thyroid diseases [29]. Unlike sex hormonebinding protein or markers of bone resorption, soluble IL-2 receptor levels are independent of gonadal status, glucocorticoid replacement, and free thyroid hormone level. It is therefore the most useful additional biochemical index of thyroid status in CH patients. The mean daily levothyroxine replacement dose at the end of the study was 1.6 g/kg in patients younger than age 60 years and 1.3 g/kg in patients older than age 60 years. This is similar to findings in subjects with primary hypothyroidism [30]. No difference was found according to sex or origin of the disease (pituitary vs. hypothalamic). Before initiating levothyroxine therapy, the need for glucocorticoid therapy should be assessed and replacement should be initiated if necessary. 3. 4. 5. 6. 7. 8. 9. 10. 11. Conclusions Central hypothyroidism is most often caused by diseases of the pituitary or hypothalamus. When the diagnosis is suspected by the finding of low FT4 and inappropriately low, normal, or slightly increased TSH, other pituitary hormone deficiencies need to be considered. The lack of a nocturnal TSH surge and a delayed TSH response to TRH support the diagnosis of CH and imaging of the pituitary is indicated. Congenital isolated CH with undetectable or low TSH is suggestive of TSH– subunit mutations, particularly when levels of glycoprotein hormone subunit are high [10]. In these patients, the hypothyroid state is not detected at neonatal screening because most centers only use TSH evaluation on a dry blood spot. This can result in a delay in diagnosis and severe hypothyroidism. A delay in treatment can result in mild to severe mental and growth retardation. Symptoms of hypothyroidism in the neonatal period necessitate an immediate comprehensive workup including molecular genetic studies, regardless of the screening results. 12. 13. 14. 15. 16. 17. 18. 19. 20. References 1. Martino E, Bartalena L, Pinchera A: Central hypothyroidism. In Werner and Ingbar’s the Thyroid edited by Braverman LE, p. 762. Philadelphia, Lippincott Williams & Wilkins 2000. 2. Persani L, Borgato S, Romoli R, et al.: Changes in the degree of sia222 21. 22. lylation of carbohydrate chains modify the biological properties of circulating thyrotropin isoforms in various physiological and pathological states. J Clin Endocrinol Metab 1998; 83: 2486–92. Samuels MH, Ridgway EC: Central hypothyroidism. Endocrinol Metab Clin North Am 1992; 21: 903–19. Ferreti E, Persani L, Jaffrain-Rea M, et al.: Evaluation of the adequacy of levothyroxine replacement therapy in patients with central hypothyroidism. J Clin Endocrinol Metab 1999; 84: 924–9. Oliveira JHA, Persani L, Beck-Peccoz P, et al.: Investigating the paradox of hypothyroidism and increased serum thyrotropin (TSH) levels in Sheehan’s syndrome: characterization of TSH carbohydrate content and bioactivity. J Clin Endocrinol Metab 2001; 86: 1694–9. Constine LS, Woolf PD, Cann D, et al.: Hypothalamic-pituitary dysfunction after radiation for brain tumors N Engl J Med 1993; 328: 87. Rose SR, Lustig RH, Pitukcheewanont P, et al.: Diagnosis of hidden central hypothyroidism in survivors of childhood cancer. J Clin Endocrinol Metab 1999; 84: 4472–9. Webb SM, Rigla M, Wagner A, et al.: Recovery of hypopituitarism after neurosurgical treatment of pituitary adenomas. J Clin Endocrinol Metab 1999; 84: 3696–700. Medeiros-Neto G, Lacerda L, Wondisford FE: Familial congenital hypothyroidism caused by abnormal and bioinactive TSH due to mutations in the beta-subunit gene. Trends Endocrinol Metab 1997. Bonomi M, Proverbio MC, Weber G, et al.: Hyperplastic pituitary gland, high serum glycoprotein hormone alpha-subunit, and variable circulating thyrotropin (TSH) levels as hallmark of central hypothyroidism due to mutations of the TSH beta gene. J Clin Endocrinol Metab 2001; 86: 1600–4. Hayashizaki Y, Hiraoka Y, Tatsumi K, et al.: Deoxyribonucleic Acid Analyses of Five Families with Familial Inherited Thyroid Stimulating Hormone Deficiency. J Clin Endocrinol Metab 1990; 71: 792–6. Medeiros-Neto G, Herodotuou DT, Rajan S, et al.: A circulating, biologically inactive thyrotropin caused by a mutation in the beta subunit gene. J Clin Invest 1996; 97: 1250–5. Dacou-Voutetakis C, Feltquate DM, Drakopoulou M, et al.: Familial hypothyroidism caused by a nonsense mutation in the thyroidstimulating hormone beta-subunit gene. Am J Hum Genet 1990; 46: 988–93. Doeker BM, Pfaffle RW, Pohlenz J, et al.: Congenital central hypothyroidism due to a homozygous mutation in the thyrotropin beta-subunit gene follows an autosomal recessive inheritance. J Clin Endocrinol Metab 1998; 83: 1762–5. Biebermann H, Liesenkotter KP, Emeis M, et al.: Severe congenital hypothyroidism due to a homozygous mutation of the betaTSH gene. Pediatr Res 1999; 46: 170–3. Heinrichs C, Parma J, Scherberg HN, et al.: Congenital central isolated hypothyroidism caused by a homozygous mutation in the TSH-beta subunit gene. Thyroid 2000; 10: 387–91. Pfaffle RW, DiMattia GE, Parks JS, et al.: Mutation of the POUspecific domain of Pit-1 and hypopituitarism without pituitary hypoplasia. Science 1992; 257: 1118–21. Wu W, Cogan JD, Pfaffle RW, et al.: Mutations in PROP1 cause familial combined pituitary hormone deficiency. Nat Genet 1998; 18: 147–9. Dattani MT, Martinez-Barbera JP, Thomas PQ, et al.: Mutations in the homeobox gene HESX1/Hesx1 associated with septo-optic dysplasia in human and mouse. Nat Genet 1998; 19: 125–33. Thomas PQ, Dattani MT, Brickman JM, et al.: Heterozygous HESX1 mutations associated with isolated congenital pituitary hypoplasia and septo-optic dysplasia. Hum Mol Genet 2001; 10(1): 39–45. Netchine I, Sobrier ML, Krude H, et al.: Mutations in LHX3 result in a new syndrome revealed by combined pituitary hormone deficiency. Nat Genet 2000; 25: 182–6. Pfaffle RW, Blankenstein O, Wuller S, et al.: Combined pituitary Volume 12, Number 3 Central Hypothyroidism 23. 24. 25. 26. hormone deficiency: role of Pit-1 and Prop-1. Acta Paediatr 1999; 88(S433): 33–41. Collu R, Tang J, Castagne J, et al.: A novel mechanism for isolated central hypothyroidism: inactivating mutations in the thyrotropinreleasing hormone receptor gene. J Clin Endocrinol Metab 1997; 82: 1561–5. Gharib H, Abboud CF: Primary idiopathic hypothalamic hypothyroidism. Am J Med 1987; 83: 171–4. Sherman SI, Jayashree G, Haugen BR, et al.: Central hypothyroidism associated with retinoid X receptor-selective ligands. N Engl J Med 1999; 340: 1075–9. Fisher DA: Management of congenital hypothyroidism. J Clin Endocrinol Metab 1991; 72: 523–9. The Endocrinologist 27. Persani L, Feretti E, Borgato S, et al.: Circulating thyrotropin bioactivity in sporadic central hypothyroidism. J Clin Endocrinol Metab 2000; 85: 3631–5. 28. Yamakita N, Komaki T, Takao T, et al.: Usefulness of thyrotropin (TSH)-releasing hormone test and nocturnal surge of TSH for diagnosis of isolated deficit of TSH secretion. J Clin Endocrinol Metab 2001; 86: 1054–60. 29. Koukkou E, Panayiotidis P, Thalassinos N: Serum soluble interleukin-2 receptors as an index of the biological activity of thyroid hormones in hyperthyroidism. J Endocrinol Invest 1995; 18: 253–7. 30. Sawin CT, Herman T, Molitch ME, et al.: Aging and the thyroid. Am J Med 1983; 75: 206–9 223