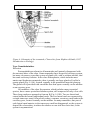
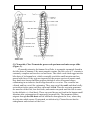
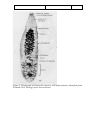
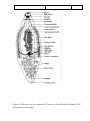
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Reproductive Organs
Survey
Document related concepts
Transcript
MINISTRY OF SCIENCE AND EDUCATION OF THE REPUBLIC OF KAZAKHSTAN
STATE UNIVERSITY Named after Shakarim, Semey
Documentof 3 level by MQS
EMСD
Educational materials on discipline
"BP in invertebrate zoology"
EMСD
#2 edition from
16.06. 2014
EMCD 042-18-35.1.31/ 03-2014
TRAINING COMPLEX OF DISCIPLINE
"BP in invertebrate zoology"
for specialty 5B011300 "Biology"
EDUCATIONAL MATERIALS ON DISCIPLINE
SEMEY
2014
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
Content
1. Glossary
2. Lectures
3. Practical and laboratory classes
4. Course work
5. Self-study of the student
p. 2 of 10
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 3 of 10
1 GLOSSARY
Abductor A muscle that moves a structure away from the middle of the body.
Abyssal The ocean bottom between 4000 and 6000 m.
Abyssobenthic The ocean bottom at depths of 4000-6000 m.
Abyssopelagic The region of the ocean's pelagic zone deeper than 4000 m.
Acanthella Acanthocephalan larva, following the acanthor and preceding the
cystacanth.
Acanthor First acanthocephalan larval stage.
Aciculum (pl. Acicula) Chitinous rod that internally supports the divisions of the
parapodium.
Acinus A small sac.
Acoelomate Body organization lacking a fluid-filled cavity between epidermis and
gastrodermis; compact.
Acontium (pl. Acontia) A thread originating from the middle lobe of an anthozoan
septal filament that projects freely into the gastrovascular cavity.
Acron The anteriormost region, preceeding the first segment of the arthropod
body.
Acrorhagus (pl. Acrorhagi) Cnidocyte-covered elevations on specialized sweeper
tentacles or on the column of certain anthozoans.
Actinotrocha Tentaculate ciliated larva of phoronids.
Actinula A polyp-like larva of certain hydrozoans that resembles a short stemless
hydranth.
Action the function of a muscle; the result accomplished by its contraction.
Adductor A muscle that moves a structure toward the middle of the body.
Adoral zone Region within the buccal cavity of certain ciliates.
Aesthetasc Chemoreceptive sensilla of crustaceans, usually on the first antenna.
Agamete Nucleus within the plasmodium of an orthonectid “mesozoan” that
divides mitotically and eventually gives rise to a sexual adult.
Allochthonous arising outside the organism or entity.
Allosperm Sperm received from a sexual partner.
Alveolus (pl. Alveoli) One of many flattened vesicles that form a more or less
continuous layer beneath the cell membrane of ciliates and a few other protozoans.
Ambulacrum (pl. Ambulacra) Groove, ridge, or double band of tube feet, radial
canal, and associated body wall of echinoderms.
Ametabolous Insect development in which the young are identical to adults except
for size and sexual maturity. No instar has wings and there is no metamorphosis.
Amictic egg The thin-shelled, parthenogenetic, diploid, rotifer egg that cannot be
fertilized and develops into amictic females. Also known as subitaneous or
summer eggs.
Ammonotelic Producing ammonia as the end product of nitrogen metabolism.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 4 of 10
Amphiblastula Sponge larva which is hollow. One hemisphere is composed of
small flagellated cells and the other is composed of large nonflagellated
macromeres.
Amphid Paired, anterior chemo- and mechanosensory organs of many nematodes.
Amphidectic In bivalves; the hinge ligament extending anterior and posterior to
the umbo.
Ampulla (pl. Ampullae) Small, muscular sac attached to an echinoderm tube foot
that bulges into the perivisceral coelom. The posterior, usually expanded, end of
the phoronid body.
Analogy Similarity resulting from evolutionary convergence rather than common
ancestry.
Anamorphic Development in which the young at hatching, have only a part of the
adult complement of segments, i.e. indirect development.
Anastomose Branching and rejoining in a complex pattern.
Ancestrula (pl. Ancestrulae) The bryozoan zooid that develops from the egg and
which produces, by cloning, all subsequent zooids of the colony.
Anisomyarian Unequal adductor muscles; resulting from reduction of the anterior
adductor.
Antenniform Shaped like an antenna, i.e. whiplike and composed of a series of
small articles.
Anoxic Without oxygen; less than 1 mg/L dissolved oxygen.
Aphotic zone Region of the ocean or a lake in which, due to insufficient light,
respiration exceeds photosynthesis.
Apical field The anterior cilia-free area surrounded by the circumapical band of
rotifers.
Apodeme An internal projection of the arthropod cuticle to which muscles are
attached.
Apomorphic Refers to an evolutionarily derived state of a homolog.
Apophysis A projection, either internal or external, of the arthropod exoskeleton.
Apopyle Outlet from a flagellated chamber to an excurrent canal in leuconoid
sponges.
Aposematic Warning coloration typical of toxic, noxious, or otherwise dangerous
species.
Arborescent Branching in a tree- or bushlike pattern.
Archenteron The embryonic gut formed during gastrulation.
Architomy Form of fission in which some planarians simultaneously fragment the
body into several pieces.
Aristotle’s lantern Highly developed chewing apparatus used for feeding by sea
urchins.
Article A section of an arthropod appendage between successive joints.
Articulate To connect by means of a joint.
Artifact Anything introduced by the process of observation and that is not a
natural part of the living organism. Also, an external product of the organism.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 5 of 10
Asconoid A sponge body that is a simple cylinder and always small.
Ascus Internal pressure-regulating sac of some cheilostome bryozoans.
Astaxanthin The red pigment in some crustaceans.
Athecate Refers to those hydroids that lack a hydrotheca.
Atoke In polychaetes showing epitoky, the non-reproductive, benthic individual.
Atoll Reef that rests on the summit of a submerged volcano.
Atrium (pl. Atria) Internal cavity through which water flows in asconoid sponges
(spongocoel). The internal cavity that receives the outflow of water from the
pharynx in hemichordates and chordates. In molluscs, the heart chamber(s)
receiving oxygenated blood from the gills; also auricle
Auricularia Primary larval stage in holothuroid development.
Autochthonous Arising within the organism or other entity.
Autogamy Nuclear reorganization without conjugation or exchange of
micronuclear material between two protozoans.
Autosperm Sperm produced by an individual.
Autotomy Self amputation. Deliberate loss of appendages, typically at specialized
fracture zones.
Autotrophic Type of nutrition in which organic compounds are obtained by
reduction of CO 2.
Autozooid Typical feeding zooids of bryozoans and some colonial anthozoans.
Avicularium (pl. Avicularia) Jawed heterozooid found in many cheilostome
bryozoans.
Axial rod Tough, collagenous endoskeleton of gorgonians.
Axil The angle between a branch or appendage and the body from which it arises.
Axopodium (pl. Axopodia) Fine, needle-like pseudopodium that contains a central
bundle of microtubules.
Axoneme Microtubules and other proteins composing the core of flagella and
cilia.
Barrier reef Reef whose platform is separated from the adjacent land mass by a
lagoon.
Basal body An organelle equivalent to a centriole at the base of flagellum or a
cilium.
Basal lamina Thin, collagenous, fibrous sheet secreted by epithelial cells and on
which they rest.
Basement membrane The layer of fibrous connective tissue under the epidermis
consisting of the basal lamina plus additional connective tissue.
Basis A bulbous, secreted structure that supports the hoplonemertean proboscis
stylet. The attached calcified floor of a sessile barnacle. The second of two basal
articles of the crustacean limb.
Bathyal The ocean bottom between 200 and 4000 m, roughly equivalent to the
continental slope.
Bathypelagic The subdivision of the pelagic zone of the ocean between 1000-4000
m.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 6 of 10
Benthic The bottom of a body of water. Organisms living on or in the bottom.
Benthos Community of organisms that lives on or in the bottom of a water body.
Bilateral symmetry Body plan in which there is a single plane of symmetry.
Binary fission Asexual division that produces two similar individuals.
Bipectinate A gill in which the filaments arise on both sides of the axis.
Biphasic A life cycle with benthic and pelagic phases.
Bipinnaria Primary free-swimming larval stage of asteroids.
Biradial symmetry Body plan with two planes of symmetry.
Biramous An annelid or arthropod appendage with two branches.
Blastaea Hypothetical ancestor that is suggested by the blastula stage which
occurs in the development of all animals.
Blastema Dome-shaped mass of unspecialized cells that forms beneath the
epidermis prior to healing and regeneration and is the source of new cells.
Blastocoel The fluid or gel-filled embryonic cavity beneath the germ layers. The
embryonic connective-tissue compartment.
Blastomere A cell resulting from the cleavage divisions of the zygote.
Blastopore Primary opening of the archenteron to the exterior of the embryo.
Blastostyle A reduced, finger-like gonozooid that bears gonophores.
Blastozooid A tunicate bud that arises from the body of the oozooid.
Blastula (pl. Blastulae) A sphere of blastomeres created by repeated cleavage
divisions of the zygote.
Blood-vascular system Circulatory system that develops within the connective
tissue.
Body ciliature Cilia distributed over the general body surface of ciliates.
Body whorl The last and largest whorl of the gastropod shell.
Bonellin Echiuran dermal pigment that may have antibiotic properties.
Brachiolaria Second asteroid larva, following the bipinnaria, marked by the
appearance of three adhesive arms at the anterior end.
Brachiole Slender, pinnule-like projection of fossil echinoderms.
Brackish Diluted sea water intermediate in salinity between sea water and fresh
water.
Branchium A gill.
Brood To care for developing eggs outside the body.
Brown body A dark sphere of waste-containing cells that remains lodged in the
coelom following regression by bryozoan polypides.
Buccal cavity Cavity just inside the mouth opening. The first region of the gut.
Buccal field A large ventral ciliated area which surrounds the mouth of some
rotifers.
Bud Protozoans: The smaller of two progeny cells resulting from fission.
Metazoans: Asexually-produced progeny that either remains attached to the parent
as a colonial zooid or undergoes differentiation before being released as a separate
individual.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 7 of 10
Bulbous pharynx Platyhelminth pharynx characterized by a sucking muscular
bulb.
Bursa (pl. Bursae) A pouchlike structure. Commonly refers to a female
reproductive chamber for the reception and temporary storage of sperm received at
copulation. The ten respiratory invaginations are at the bases of the arms of many
ophiuroids.
Byssus A bundle of secreted protein threads used to attach some bivalves to the
substratum.
Calcareous Composed of calcium carbonate.
Calymma A broad vacuolated cortex formed by extracapsular cytoplasm that
surrounds the central capsule of radiolarians.
Calyx Skeletal cup of a crinoid disc. The body and tentacles of an entoproct.
Campanulate Bell-shaped
Capitulum The body of stalked barnacles exclusive of the stalk.
Captaculum (pl. Captacula) A threadlike feeding tentacle of scaphopod molluscs.
Carapace The fold of body wall that extends posteriorly from the arthropod head
to cover some or all of the trunk.
Carina Posterior median plate of the barnacle exoskeleton. One of the five
primary plates.
Casting Continuous pile of defecated organic and mineral matter.
Caudal gland A posterior spinneret typical of many free-living nematodes.
Central capsule The membrane-enclosed, innermost cytoplasm of a radiolarian
cell.
Centriole Microscopic cylindrical structure, composed of microtubules, which is
situated at each pole of the mitotic spindle and is distributed to daughter cells
during mitosis. There it may function as a basal body and give rise to a flagellum
or cilium.
Centrolecithal Refers to an arthropod egg which gives rise to a blastula in which
the yolk is central and surrounded by peripheral cytoplasm.
Centrosome Structure from which bundles of microtubules radiate outwards.
Cephalic gland Slime secreting gland of nemerteans.
Cephalization Tendency to coalesce the segmental ganglia into a large anterior
neural center.
Cephalothorax The combined head and thorax.
Ceras (pl. Cerata) Projection from the dorsal body surface of many nudibranchs.
Cercaria (pl. Cercariae) Free-swimming developmental stage of digenean
trematodes.
Cerebral organ One of a pair of ciliated sensory canals associated with the
nemertean brain.
Cetacea Order of marine mammals containing whales and porpoises.
Chaeta A cuticular bristle composed of b -chitin.
Chain A free-swimming aggregate of sexual zooids in salps.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 8 of 10
Chelate Refers to appendages that are pincer-like consisting of movable and
immovable fingers.
Chelicera (pl. Chelicerae) The anteriormost appendages of chelicerates.
Cheliped A chelate thoracic appendage of decapod crustaceans.
Chilarium (pl. Chilaria) The appendage of the first abdominal segment of
horseshoe crabs. Chitin A polysaccharide of polymerized N-Acetylglucosamine
residues.
Chlorocruorin Type of polychaete hemoglobin that is green in color.
Chondrophore A depression in the hinge housing the inner ligament of some
bivalves.
Chorion The shell-like membrane secreted by ovarian follicle cells that surrounds
the eggs when they reach the oviduct.
Chromatophore A cell or organ that expands or contracts to alter the color of the
organism.
Cilium (pl. Cilia) Characteristic of many protozoan and metazoan cells, a motile
outgrowth of the cell surface that is typically short and its effective stroke is stiff
and oarlike.
Cingulum Dinoflagellates: horizontal or transverse groove that bears the
transverse flagellum. Rotifers: posterior (postoral) band of cilia of the divided
corona.
Circumapical band A ribbon of cilia encircling the anterior end of the rotifer
head.
Cirrus (pl. Cirri) Name given to various appendages, usually tentacle-like and
curled.
Cirrus sac Contains the internal seminal vesicle, prostate glands, and cirrus of
some platyhelminths.
Cnida (pl. Cnidae) An eversible cnidarian organelle that occurs in a cnidocyte.
Cnidocil A short, stiff, bristle-like cilium that is borne on a cnidocyte.
Cnidocyte A cnidarian cell that contains an eversible cnida.
Cnidosac Distal tip of a ceras of cnidarian-eating nudibranchs. The sac is an
extension of the gut and contains undischarged nematocysts acquired from the
prey.
Coenecium A branching tubular network inhabited by pterobranch colonies that is
secreted from glands in the oral shields of the zooids.
Coelenteron The body cavity and gut of cnidarians and ctenophores.
Gastrovascular cavity.
Coeloblastula Blastula having a well developed blastocoel.
Coelom Body cavity lined by a mesodermally derived epithelium.
Coelomate An animal having a coelom.
Coelomocyte A circulating coelomic cell which may or may not contain a
respiratory protein.
Coelomoduct A mesodermally derived duct leading from a coelom to the exterior.
Usually a gonoduct.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 9 of 10
Coenenchyme All of the tissue situated between polyps in anthozoan colonies.
Coenosarc Ther living tissue underlying the cuticular perisarc of hydroids.
Collagen Common animal fibrous protein that forms extracellular skeletal
materials.
Collar Anthozoans: Circular fold at the junction of the column and the oral disc.
Enteropneusts: The second of three body divisions.
Collencyte A fixed cell of sponges that is anchored by long, cytoplasmic strands
and secretes dispersed collagen fibers (not spongin).
Colloblast An adhesive cell situated on the tentacles of ctenophores.
Collum The first anterior, legless segment of millipedes that forms a collar behind
the head.
Colony Body composed of structurally joined zooids that share resources.
Columella Central axis of asymmetrical shells around which whorls are coiled.
Columnar epithelium Epithelium of elongated cells.
Comb A flat paddle of fused cilia in ctenophores.
Comb row One of eight ciliary bands of ctenophores, each composed of a series of
combs.
Commensalism A type of symbiotic relationship in which one species benefits
from the relationship and the other species (host) is neither benefited nor harmed.
Commissure A more or less transverse nerve that joins the two ganglia of a pair.
Compact Body without a large fluid filled space: acoelomate.
Complemental male A male barnacle that develops attached to a hermaphrodite
individual.
Complete cleavage Cleavage furrows extend completely through the egg mass;
holoblastic.
Compound eye An arthropod eye composed of multiple ommatidia.
Compressed Flattened laterally, from side to side.
Conchiferan “Shell-bearers”, includes monoplacophoran, gastropod, bivalve,
scaphopod, and cephalopod molluscs.
Conchiolin The secreted molluscan protein of which the periostracum, byssus, and
operculum are composed.
Confamilial Belonging to the same family.
Congeneric Belonging to the same genus.
Conjugant One of a pair of fused ciliates in the process of exchanging genetic
material.
Connective A more or less longitudinal nerve that connects two ganglia of
different pairs.
Connective tissue Body layer between epithelia, composed of a fluid or gel
extracellular matrix with or without cells.
Connective tissue compartment ··Body layer occupied by connective tissue.
Conspecific Belonging to the same species.
Contractile vacuole Large spherical vesicle responsible for osmoregulation in
protozoans and some sponge cells.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 10 of 10
Contractile vacuole complex Protozoan system of water and ion pumping
organelles.
Convergence Independent evolution of similar structures.
Copraphagy Ingestion of feces.
Coracidium A ciliated free-swimming developmental stage of cestodes.
Cordate Heart-shaped.
Corona Ciliated organ at anterior end of rotifers used for feeding and swimming.
Cortex An outer ectoplasmic layer.
Cosmopolitan Worldwide distribution.
Coxa (pl. Coxae) The proximal article of an arthropod appendage.
Cryptobiosis A desiccated, metabolically inactive, resistant condition.
Ctenidium (pl. Ctenidia) A molluscan gill.
Cuboidal epithelium Epithelium in which the cells are roughly cubical in shape.
Cursorial Running.
Cuticle Protective or supportive, nonliving, external, layer secreted by the
epidermis.
Cyclomorphosis Seasonal changes in body shape or proportions.
Cydippid A free-swimming ctenophore larva having an ovoid or spherical body.
Cyphonautes Planktotrophic larva of some species of nonbrooding gymnolaemate
bryozoans.
Cypris An ostracod-like, settling larval stage of barnacles.
Cysticercus Developmental stage of certain tapeworms, following the oncosphere,
and characterized by a fluid-filled oval body with an invaginated scolex.
Cystid The exoskeleton and body wall of bryozoans.
Cytopharynx Permanent oral canal, or passageway, of ciliates that is separated
from the cytoplasm by the cell membrane.
Cytoproct Permanent cellular anus of some ciliates.
Cytostome Cell mouth.
Dactylozooid A finger-shaped, defensive, hydrozoan polyp.
Dedifferentiation Loss of specialized cellular features returning to a more
generalized condition. Characteristic of certain aspects of development, especially
regeneration.
Definitive host The host for the adult stage of a parasite.
Dendritic Treelike
Dendrobranchiate Having bushy, branching gills.
Deploying point A site of separation of an asexually-produced group of salp
blastozooids from other such groups.
Deposit feeding Feeding upon detritus that has settled to the bottom of aquatic
environments.
Depressed Flattened dorsoventrally.
Derived Changed evolutionarily from the ancestral condition.
Determinate cleavage Developmental process during which the fates of the
blastomeres are fixed early in cleavage; mosaic development.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 11 of 10
Detritus Fragments of dead plants or animals.
Deuterostome Member of a major branch of the animal kingdom in which the site
of the blastopore is posterior—far from the mouth, which forms as a new opening
at the anterior end.
Diapause A period of arrested metabolism to survive adverse environmental
conditions.
Diastole The relaxation, or dilation, phase of a heart beat.
Dicondylic Articulated by two movable hinges, or condyles.
Dioecious Having separate sexes; gonochoric.
Digitiform Finger-shaped.
Dimorphism Exhibiting two shapes or appearances.
Diotocardian Heart with two atria.
Diploblastic With only two embryonic germ layers.
Diplosegment Double trunk segments derived from the fusion of two separate
segments.
Direct deposit feeding (non-selective deposit feeding) Indiscriminate ingestion of
mixed organic and mineral particles with no selection or sorting prior to entry into
the mouth.
Direct development Lacking a larval stage. On hatching the young have the adult
body form.
Directive Either of two pairs of septa at each edge of the compressed anthozoan
pharynx.
Distal Distant from the center, origin, or midline.
Diurnal Active during the day.
Diverticulum An outpocketing or pouch, cecum.
Doliolaria Barrel-shaped larval stage, following the auricularia, of holothuroids.
Dormant egg Anegg capable of adverse conditions for long periods before
hatching.
Dorsal lamina Longitudinal tissue fold along the inner dorsal pharyngeal wall of
some ascidians. Gathers mucous net and food and conveys them into the
esophagus.
Duogland Secretory system consisting of cells producing an adhesive and others
producing a compound to inactivate the adhesive.
Dwarf male A male reduced in size through degeneracy or loss of structures.
Ecdysis The periodic loss of the exoskeleton; molting.
Ecdysone Hormone that promotes molting.
Echinopluteus Planktotrophic larva of echinoid echinoderms that bears six pairs
of long larval arms.
Eclosion Emergence of the imago from the pupa or last nymphal cuticle but
sometimes, confusingly used as a synonym for hatching from the egg.
Ectoderm Embryonic germ layer composing the outer wall of the gastrula.
Ectoparasite Parasite that lives on the outside of its host.
Ectosymbiont An symbiont living outside its host.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 12 of 10
Electron dense Appearing dark in electron photomicrographs.
Electron lucid Appearing clear in electron photomicrographs.
Elytrum (pl. Elytra) Platelike scale, modified from a dorsal cirrus, that is borne on
a short stalk on the dorsal side of the body of scaleworm polychaetes.
Embryonated egg An embryo, rather than an ovum, enclosed in an egg shell.
Encystment Forming resistant cysts in response to unfavorable conditions such as
lack of food or desiccation.
Endemic Species found in a restricted geographic area and nowhere else.
Endocytosis Process in which some extracellular materials enter a cell in minute
pits on the cell’s membrane that later pinch off internally.
Endoderm Embryonic germ layer composing the archenteron wall.
Endogastric coiling The shell coils posteriorly, over the foot.
Endoparasite Parasite that lives inside its host.
Endosymbiont A symbiont living inside its host.
Endoral membrane Ciliate undulating membrane that runs transversely along the
right wall and marks the junction of the vestibule and buccal cavity.
En face Head on.
Enterocoel Coelomic cavity formed from an outpocketing of the embryonic
archenteron.
Enteronephric Refers to either typical or modified nephridia that open into
various parts of the digestive tract of earthworms.
Enzymatic-gland cell Cell responsible for the secretion of digestive enzymes into
the cnidarian coelenteron.
Ephemeral Short-lived, brief.
Ephippium (pl. Ephippia) A resistant egg capsule formed in the cladoceran brood
chamber.
Ephyra (pl. Ephyrae) An immature scyphomedusa.
Epiathroid Nervous system in which the cerebral and pleural ganglia are
contiguous.
Epibenthic Living on or just above the bottom of an aquatic habitat.
Epiboly Type of morphogenetic movement in gastrulation in which ectodermal
cells overgrow the inner germ layers.
Epicuticle Thin, outer, proteinaceous layer of the arthropod skeleton.
Epidermal replacement cell A platyhelminth parenchymal cell that migrates from
the parenchyma to the body surface and replaces a damaged or destroyed
epidermal cell.
Epidermis Outer epithelial layer of the body.
Epifauna The animals that live on the surface of ocean, lake, and stream bottoms.
Epigastric coiling The shell coils anteriorly, over the head.
Epigean Above ground.
Epimorphic Development in which the young hatch with the full complement of
segments, i.e. direct development.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 13 of 10
Epipelagic The uppermost layer, to a depth of 200 m, of the pelagic zone, roughly
equivalent to the euphotic zone.
Epiphytic Living on the surface of a plant.
Epiplasm Dense supportive mesh formed by filamentous proteins in the cortical
cytoplasm.
Epitheliomuscular cell A cnidarian contractile cell that has characteristics of both
epithelial and muscular cells.
Epitoky Reproductive phenomenon in some polychaetes: the production, either by
transformation or budding, of a reproductive individual (epitoke) adapted for a
pelagic existence from a nonreproductive individual adapted for a benthic
existence.
Epizoic Living on the surface of an animal.
Equilateral Anterior and posterior ends of a bivalve valve are of similar shape and
size.
Equivalve The two valves of a clam being the same size and shape.
Esthete A sensory organ in a minute vertical canal in the upper layer of the chiton
shell plate.
Estivation (= aestivation)A dormant state in which some animals pass hot, dry
seasons.
Estuary Embayment at the junction of a river with the sea, typically with brackish
water.
Eukaryotic A cell with membrane-bound organelles including nucleus and
mitochondria.
Eulamellibranch gill Bivalve gill with filaments joined together by continuous
sheets of tissue.
Euphotic zone Upper layer of water, 0-100 m depending on turbidity, in which
there is sufficient light to support photosynthesis in excess of respiratory needs.
Eutely Having an invariant, species-specific, and genetically-fixed number of cells
or nuclei.
Euthyneury Symmetrical, untwisted, detorted, gastropod nervous system.
Euryhaline Tolerant of a wide range of environmental salinities.
Evert Protrusion by turning inside out.
Evisceration When the anterior or posterior end of a species ruptures and parts of
the gut and associated organs are expelled.
Exconjugant Ciliates that have separated after sexual reproduction.
Exocytosis Process in which indigestible material is released from a cell to the
exterior by fusion of the residual vesicle with the cell membrane.
Extrinsic A muscle extending from one structure to another.
Exumbrella Aboral, upper surface of the bell of a medusa.
Fasciole One of several ciliated spines of certain echinoids that together form a
siphon.
Filibranch gill Bivalve gill in which filaments are held together by tufts of cilia.
Filiform Having the shape of a filament or thread.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 14 of 10
Filopodium (pl. Filopodia) Pseudopodium that is slender, clear, and sometimes
branched.
Filter feeding A type of suspension feeding in which organic particles (plankton
and detritus) are removed from a water current by a filter.
Fin box One in a longitudinal series of small, median, unpaired coelomic cavities
that form and help to support the dorsal and ventral fins of cephalochordates.
Fin ray Any of several stiff, slender structures that support a fin.
Fission Asexual division of an organism into two or more progeny.
Fixed parenchymal cell A large, branched, mesodermal cell of platyhelminths
that makes contact with and interjoins other cells and tissues.
Flagellum (pl. Flagella) A characteristic of many protozoan and metazoan cells; it
is typically long and its motion is a complex whip-like undulation.
Flame cell A protonephridial terminal cell that has many flagella, which beat
synchronously and resemble a minute flickering flame; its nucleus is at the base of
the flame. Flame bulb.
Flosculi Cuticular sensory structures consisting of monociliated cells with a collar
of microvilli.
Foliaceous Erect, leaflike, bryozoan colony composed of one or two sheets of
zooids.
Food vacuole Cellular vesicle containing ingested food.
Foot Muscular, flattened, ventral surface of a mollusc, forming a creeping sole.
Forcipule Appendage of the first centipede trunk segment; poison claw.
Fossorial Adapted for digging.
Free living 1. Not parasitic. 2. Not permanently attached to a substratum.
Fringing reef Reef that extends seaward directly from the shore.
Frontal gland Anterior aggregation of secretory cells in platyhelminths.
Fruticose Erect bushlike bryozoan colony.
Funiculus (pl. Funiculi) A mesothelial cord extending across the bryozoan
coelom.
Fusiform Spindle- or cigar-shaped, i.e. thick in the middle and tapered bluntly at
both ends.
Gamogony Multiple fission that forms gametes that fuse to form a zygote.
Ganglion (pl. Ganglia) An aggregation of neuronal cell bodies.
Gap junction Intercellular junction that allows for intercellular communication,
such as electrical coupling of muscle cells.
Gastric filament One of several cnidocyte-bearing threads that extend into the
scyphozoan stomach from the septa between gastric pockets.
Gastric mill Part of the malacostracan cardiac stomach where food is triturated by
internal teeth.
Gastric pouch or pocket One of four pockets in the wall of the scyphozoan
stomach.
Gastrodermis Cellular epithelial lining of the gastrovascular cavity of cnidarians
and ctenophores and the midgut lining of bilaterally symmetrical animals.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 15 of 10
Gastrolith A calcareous concretion in the stomach of some crustaceans for
calcium storage.
Gastrovascular cavity Internal extracellular cavity of cnidarians and ctenophores
lined by gastrodermis.
Gastrozooid Nutritive or feeding polyp of cnidarians which is similar to a short
hydra.
Gastrula A two-layered embryo.
Gastrulation The developmental establishment of germ layers.
Geniculate Bent at a sharp angle, like an elbow.
Genital atrium A small chamber in parasitic platyhelminths that receives the
openings of both the male and female reproductive systems.
Gestate To care for developing eggs inside the maternal body.
Gill An outward expansion of the body surface for the purpose of gas exchange in
water.
Girdle The thick, stiff, peripheral area of the chiton mantle laterally beyond the
shell plates.
Glycocalyx (pl. Glycocalyces) The carbohydrate and protein surface coat of
eukaryotic cells.
Gnathobase Spiny medial surface of the basal articles of many arthropod limbs.
Gnathochilarium A broad flattened plate formed of a fused pair of maxillae in
millipedes.
Gnathopod Each of the second and third thoracic appendages of amphipods.
Gonangium (pl. Gonangia) Type of gonozooid that consists of a central
blastostyle bearing gonophores and is surrounded by an extension of the perisarc
(gonotheca).
Gonochoric Separate sexes (dioecious).
Gonoduct Principal duct providing for the transport of sperm or eggs in any
reproductive system.
Gonophore A hydroid reproductive bud that bears the germ cells and may become
a free-swimming medusa or a variously modified sessile medusa. Medusoid.
Gonopore External opening of any reproductive system.
Gonotheca (pl. Gonothecae) An extension of the perisarc around a gonozooid.
Gonozooid A hydrozoan reproductive polyp which is often reduced, lacking
mouth and tentacles, and bears gonophores. A sexually reproductive zooid of
thaliaceans.
Gorgonin A tanned collagen.
Gravid Bearing developmental stages, such as eggs or embryos, internally.
Gross Large scale, not fine or delicate, i.e., not microscopic or ultrastructural.
Facies A characteristic shape or appearance.
Growth zone Region that includes all of the larva between the mouth and
telotroch on the fully developed trochophore larva.
Hadal The deep oceanic trenches at depths greater than 6000 m.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 16 of 10
Halteres Reduced second pair of dipteran (fly) wings, functions as a gyroscope to
maintain stability in flight.
Haptocyst Special adhesive organelle borne on the tentacles of suctorians.
Haptor Attachment organ that bears hooks and suckers.
Hatschek’s groove A shallow ciliated invagination of the dorsal wall of the
vestibule of cephalochordates.
Hemal system Blood-vascular system.
Hemimetabolous Insectdevelopment characterized by nymphs that do not closely
resemble adults but that do not undergo a radical metamorphosis.
Hemocoel A voluminous, blood-filled cavity, occupying much of the body.
Hermaphroditic Having both male and female reproductive systems in the same
individual. When both systems are present at the same time, the hermaphroditism
is said to be simultaneous; when the male system appears and functions first and is
followed by the female system, the hermaphroditism is said to be protandric.
Heterogony Alternating sexual and asexual phases in a life cycle.
Heteronomy Segments and appendages regionally specialized.
Heterotrophic Nutrition in which organic compounds are obtained by consuming
other organisms.
Heterozooid Modified bryozoan zooids that have functions other than feeding.
Higgins larva Larval stage of loriciferans.
Hinge ligament A noncalcified, elastic, proteinaceous band joining the two valves
of a bivalve.
Holoblastic cleavage Cleavage furrows extend completely cut through the egg
mass.
Holometabolous Insect development in which larvae and adults are distinctly
different and a major metamorphosis is required to transform the juvenile into the
adult.
Holonephridium (pl. Holonephridia) A typical, segmental metanephridial duct of
an oligochaete.
Holoplankton Plankters that spend the entire life cycle in the plankton.
Holothurin Toxic substance released in the Cuvierian tubules of certain
holothuroids.
Homolecithal egg Egg in which the yolk is uniformly distributed. Isolecithal.
Homology Similarity of structure attributable to common ancestry in two or more
species.
Homolog A characteristic a species that shares a common genetic, evolutionary,
and developmental origin with a characteristic in another species.
Homonomy All segments and appendages alike, without regional specialization.
Hyaline T ranslucent or transparent, clear.
Hydranth The oral end of a hydroid polyp bearing the mouth and the tentacles.
Hydrocaulus The stalk of a hydroid polyp.
Hydrocoral Colonial, calcified polypoid hydrozoan with either an encrusting or an
upright growth form.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 17 of 10
Hydroid colony A collection of polyps in which each polyp is connected to the
others.
Hydromedusa (pl. Hydromedusae)··Hydrozoan medusa.
Hydrorhiza (pl. Hydrorhizae) Horizontal rootlike stolon of a hydroid colony that
grows over the substratum.
Hydrotheca (pl. Hydrothecae) A cuticle that encloses the hydranth. Theca.
Hyperparasitism A parasite parasitized by another parasite.
Hyperstrophic coiling Larval protoconch is coiled at right angles to the postlarval teloconch.
Hypoathroid Nervous system with the pedal and pleural ganglia contiguous.
Hypobranchial gland Mucus-secreting epithelium on the molluscan mantle roof.
Hypogean Subterranean, below ground.
Hypognathus Insect head orientation that causes the mouthparts to be directed
downward.
Hypostome A mound or cone that bears the mouth of hydropolyps. Manubrium.
Hypoxia Less than 2 mg/L of dissolved oxygen
Imago The final instar, or adult stage, of an insect life cycle.
Incident light In microscopy, light striking the object from above the stage.
Incomplete cleavage Cleavage furrows do not completely cut through the egg
mass; meroblastic.
Incurrent canal Tubular invagination of the sponge pinacoderm that leads into the
flagellated chambers.
Incurrent pore Small opening on the surface of sponges that leads into an
incurrent canal. Ostium.
Indeterminate cleavage Fate of the blastomeres is fixed relatively late in
development. Regulative development.
Indirect deposit feeding (selective deposit feeding)Use of appendages, tentacles,
or cilia to select organic particles for ingestion.
Indirect development Having a larval stage(s) between egg and adult.
Inequilateral anterior and posterior ends of a bivalve valve are dissimilar.
Inequivalve The two valves of a clam of different sizes.
Infauna Animals that live within bottom sediments.
Infraciliary system The entire assemblage of ciliary basal bodies, or kinetosomes,
and the fibers that link them together in the cell cortex of ciliates.
Infusoriform larva The final free-swimming larval stage of rhombozoans.
Ingression Mode of gastrulation in which cells of the blastula wall proliferate cells
into the blastocoel.
Insertion One of the two attached ends of a muscle. Of the two, the insertion is
usually distal and moves when the muscle contracts.
Instar Each of the several stages between successive ecdysozoan molts.
Integument The outer layers of the body wall. Usually comprising the epidermis
and underlying connective tissue (dermis) plus any secreted cuticle or exoskeleton.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 18 of 10
Intercellular junction Membrane specialization that binds cells together,
promotes communication between cells, or helps to regulate transport across an
epithelium.
Intermediate host The host for larval stages of a parasite.
Interstitial cell A small, rounded totipotent cnidarian cell, sandwiched between
cells of the epidermis and gastrodermis.
Interstitial fauna Animals that live in the spaces between sand grains.
Intertidal The coastline between the low and high tide levels, also known as the
littoral zone.
Intrinsic Confined within a structure; not extending to neighboring structures.
Introvert Eversible part of the bryozoan, priapulid, or sipunculan body.
Invagination Infolding. In gastrulation, this refers to a type of morphogenetic
movement in which the cells of the vegetal hemisphere fold into the interior to
form the archenteron.
Isolecithal egg Egg in which the yolk is uniformly distributed. Homolecithal.
Isomyarian Anterior and posterior adductor muscles approximately equal in size..
Jellyfish A cnidarian medusa.
Kairomone A substance released by a predator to which the prey may respond
defensively.
Kenozooid A bryozoan heterozooid modified to form a stolon.
Kinetodesma (pl. Kinetodesmata) A fine striated fiber that connects kinetosomes
(basal bodies) of ciliates.
Kinetoplast Conspicuous mass of DNA that is situated within the single, large,
elongated mitochondrion of kinetoplastid (trypanosome) protozoans.
Kinetosome A ciliary or flagellar basal body.
Kinety (pl. Kineties) One row of cilia, kinetosomes, and kinetodesmata of ciliates.
Lacustrine Pertaining to lakes.
Lamella (pl. Lamellae) A sheet or flat plate of tissue. In bivalves, each of the gill
surfaces.
Languet One of several folds of tissue along the dorsal pharyngeal wall of some
ascidians which together convey food to the esophagus. A discontinuous dorsal
lamina.
Lappet Lobe formed by the margin of the scalloped scyphozoan bell. Movable
flaps that can expose or cover the ambulacral groove of crinoids.
Larva (pl. Larvae) An independent, motile, developmental stage that does not
resemble the adult.
Larviparous Eggs brooded internally within the female that are later released as
larvae.
Lateral canal Part of the echinoderm water-vascular system that joins the radial
canal and tube feet.
Laurer’s canal Short, inconspicuous canal that extends from the seminal
receptacle of trematodes to the dorsal surface, where it may open at a minute pore.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 19 of 10
Lecithotrophic brooding Viviparous development in which the embryo is
nourished by yolk.
Lecithotrophic larva A nonfeeding larva that utilizes yolk as a source of nutrition.
Lemniscus (pl. Lemnisci) Fluid filled invaginations of unknown function in
acanthocephalans.
Leuconoid Refers to a type of sponge body organization built around flagellated
chambers and an extensive system of canals.
Ligament sac A connective-tissue sac in the acanthocephalan hemocoel containing
the gonads.
Littoral In the sea; synonymous with intertidal. In lakes; the nearshore zone in
which light sufficient to support rooted vegetation reaches the bottom.
Lobopodium (pl. Lobopodia) A pseudopodium that is rather wide with rounded or
blunt tips, is commonly tubular, and is composed of both ectoplasm and
endoplasm.
Longitudinal cord Ridge that entends the length of the body created by the inward
expansion of the epidermis in nematodes and some gastrotrichs.
Lophophoral organ An area on the phoronid lophophore where spermatophores
are formed.
Lophophore A circular or horseshoe-shaped fold of the mesosomal body wall
encircling the mouth, bearing hollow ciliated tentacles, and excluding the anus.
Lorica In rotifers; an intracytoplasmic skeleton.
Luciferase Enzyme that catalyzes the bioluminescence reaction.
Luciferin The substrate of luciferin capable of bioluminescence.
Lunule One of the large, elongated notches or openings in the bodies of some
clypeasteroids (sand dollars).
Lyriform organ Group of slit sense organs found on some arachnids.
Macerate To soften and separate the parts of a solid object.
Macromere One of several large blastomeres located in the yolky vegetal
hemisphere of early embryos.
Macronucleus (pl. Macronuclei Large, usually polyploid, ciliate nucleus
concerned with the synthesis of RNA, as well as DNA, and therefore directly
responsible for the phenotype of the cell.
Macrophagous Collecting and ingesting large food particles.
Madreporite Pore or sieve plate of the echinoderm water-vascular system that
connects the stone canal to the exterior seawater (most echinoderms) or to the
perivisceral coelomic fluid (crinoids and holothuroids).
Malpighian tubule Excretory system consisting of a blind, tubular, contractile,
excretory evagination of the arthropod midgut.
Manca larva A peracarid postlarva that has all appendages except the eighth
thoracopods.
Mangrove A small tropical tree or large shrub adapted for living in the intertidal
zone.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 20 of 10
Mantle A body wall fold that secretes a shell, as in molluscs, barnacles, and
brachiopods. The body wall beneath the ascidian tunic.
Mantle cavity Protective chamber created by the overhang of a mantle; pallial
cavity.
Manubrium (pl. Manubria)··Tubelike extension, bearing the mouth, that hangs
down from the center of the subumbrella of cnidarian medusae. Hypostome of
hydroid polyps.
Marsupium (pl. Marsupia) Brood pouch outside the body.
Mastax The cuticular pharyngeal jaw apparatus of a rotifer.
Mastigoneme One of the many fine, lateral branches of some flagella.
Mastigont system Complex formed by groups of flagella and several microtubular
and fibrillar organelles.
Matrotrophic brooding Viviparous development in which the embryo is
nourished by the mother.
Medulla Central part of the heliozoan cell that is composed of dense endoplasm,
containing one to many nuclei and the bases of the axial rods.
Medusa (pl. Medusae) Form of cnidarian that has a well developed, gelatinous
mesoglea and is generally free-swimming.
Megalops Crab postlarva with a large abdomen and full complement of
appendages.
Megasclere A large spicule forming one of the chief supporting elements in the
skeleton of sponges.
Mehlis’s gland Conspicuous unicellular gland cells associated with the
reproductive system of trematodes which play a role in egg capsule formation.
Meiofauna Small metazoans that pass through a 1 mm sieve but are retained by a
mesh of 42 µm; usually referring to those living in small confined spaces.
Membranelle Type of ciliary organelle derived from two or three short rows of
cilia, all of which adhere to form a more or less triangular or fan-shaped plate that
beats as a unit.
Meroblastic Cleavage furrows do not completely cut through the egg mass.
Meroplankton Plankters that spend only part of the life cycle in the plankton.
Merozoites Individuals produced by multiple fission of sporozoan trophozoites.
Mesenchyme A network of loosely associated, often motile, embryonic cells, that
are usually, but not always, of mesodermal origin. The term is still applied to adult
connective tissues of some groups of animals.
Mesentery (pl. Mesenteries) A longitudinal sheet of tissue that divides the body
cavity of bilaterally-symmetrical animals.
Mesentoblast Blastomere associated with spirally cleaving zygotes that contains
an unidentified cytoplasmic factor which causes the cell and its progeny to form
mesoderm.
Mesoderm Embryonic germ layer that forms the tissues situated between
ectoderm and endoderm.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 21 of 10
Mesoglea Connective-tissue layer between the epidermis and gastrodermis of
cnidarians and ctenophores.
Mesohyl Sponge connective tissue. Lies beneath the pinacoderm and consists of a
gelatinous proteinaceous matrix containing skeletal material and ameboid cells.
Mesopelagic Subdivision of the pelagic zone, 200-1000 m.
Mesothelium (pl. Mesothelia) Single, nonstratified epithelium lining the coelom.
Metacercaria (pl. Metacercariae) Encysted final stage of digenean development.
Metachrony Wave pattern that results from the sequential coordinated action of
cilia or flagella over the surface of a cell or organism.
Metamere A body segment or somite.
Lacunar canal system Acanthocephalan circulatory system within the syncytial
epidermis.Metamerism Segmentation;division of the body into a linear series of
similar modules.
Metamorphosis (pl. Metamorphoses)··Transformation from a larva into an adult.
Metanauplius (pl. Metanauplii) One of several instars following the crustacean
nauplius.
Metanephridial system Excretory system composed of a vascular ultrafiltration
site, a coelomic space, and a metanephridium tubule.
Metanephridium (pl. Metanephridia) An excretory tubule that opens into the
coelom by a ciliated funnel and to the exterior by a nephridiopore.
Metatroch A second girdle of cilia that develops posterior to the prototroch of a
trochophore.
Micromere One of many small blastomeres located in the animal hemisphere of
the cleaving zygote.
Micronucleus (pl. Micronuclei)··Small, usually diploid, ciliate nucleus concerned
primarily with the synthesis of DNA. It undergoes meiosis before functioning in
sexual reproduction.
Microphagous Specialized for feeding on small food particles.
Micropyle An opening in the eggshell or resting stage from which the primordium
eventually emerges.
Microsclere A tiny sponge spicule.
Microtrich Type of microvillus found on the tegument of tapeworms.
Microtubule organizing center (MTOC) A region around basal bodies and
centrioles that controls the organized assembly of microtubules.
Mictic egg Type of fertilized rotifer egg that is thin-shelled, haploid, and can be
fertilized.
Milieu Environment.
Miracidium (pl. Miracidia) Ciliated, free-swimming, first larva of digenean
trematodes.
Molt To shed the old cuticle as a new cuticle is being secreted. Ecdysis.
Monocondylic Articulated by one movable hinge (condyle).
Monolayered epithelium Consisting of a single layer of cells resting on a basal
lamina (= simple epithelium).
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 22 of 10
Monomyarian Bivalve condition in which the anterior adductor muscle is lost.
Monopectinate Refers to a gill in which the filaments occur on only one side of
the axis.
Monophyletic group All species descended from a common ancestor.
Monospecific A taxon consisting of a single species.
Monotocardian Heart with one atrium.
Monotypic A taxon consisting of a single species.
Mosaic development Embryonic fate determination in which cell fate is
determined early in development and is the result of the action of specific factors
that are unevenly distributed, like pieces of a mosaic, in the cytoplasm of the
uncleaved egg.
Mucocysts Mucigenic bodies that are arranged in rows, similar to ciliate
trichocysts, and discharge a mucoid material.
Mucus Animal secretion utilized in a variety of ways as an adhesive, protective
cover, or lubricant.
Mutualism A symbiotic relationship in which both species benefit.
Myocyte Type of sponge mesohyl cell which displays some similarities to a
smooth muscle cell in shape and contractility. A muscle cell.
Myoepithelial cell A muscle cell that is part of an epithelium.
Myogenic Originating in a muscle cell.
Myoneme A bundle of contractile filaments that lies in the pellicle of some
protozoans.
Nacre The smooth, lustrous, usually innermost, shell layer of some molluscs;
mother of pearl.
Natatory Adapted for swimming.
Naupliar eye Median crustacean eye composed of three or four ocelli.
Nauplius Earliest hatching stage and basic crustacean larva; has three pairs of
appendages.
Neap tides Tides occurring on quarter moons characterized by modest tidal
amplitudes.
Nectophore Mouthless, pulsating swimming bell of siphonophores.
Nematocyst Stinging cnida of cnidarians.
Nematodesma (pl. Nematodesmata) One of several microtubular rods that line and
support the wall of the ciliate cytopharynx and assist in the inward transport of
food vacuoles.
Nematogen Adult dicyemid.
Neoblast A totipotent cell that is important in wound healing and regeneration.
Nephridium (pl. Nephridia) An excretory tubule usually opening to the exterior
via a nephridiopore. See protonephridium, metanephridium.
Nephrocyte A large phagocytic cell, alone or in clusters, in the hemocoel of many
arthropods.
Nephromyces A unicellular fungus that occurs in the renal sacs or the pericardium
of some ascidians.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 23 of 10
Nephrostome An open ciliated funnel at the inner, coelomic, end of a
metanephridium.
Neritic zone The water above the continental shelves.
Nerve net Plexus.
Neurogenic Originating with a neuron.
Neuropil A concentration of axons and synapses in a ganglion.
Neuropodium (pl. Neuropodia) The ventral branch of a polychaete parapodium.
Niche An organism's role in its ecosystem.
Nocturnal Circadian behavior characterized by activity at night.
Non-selective deposit feeding See direct deposit feeding.
Notopodium (pl. Notopodia) The dorsal branch of a polychaete parapodium.
Nuchal organ One of a pair of ciliated chemosensory pits or slits that are often
eversible and are situated in the head region of most polychaetes.
Nutritive-muscle cell A muscle cell in the cnidarian gastrodermis that usually
bears a cilium and is responsible for intracellular digestion.
Obturaculum (pl. Obturacula) One of two elongated, medially fused structures
which arise anteriorly from the head of vestimentiferan pogonophores and bear and
support the gills.
Occluding junction Sealing junction between cells.
Oceanic zone The division of the pelagic realm seaward of the continental shelf.
Ocellus (pl. Ocelli) A simple eye.
Odontophore A muscular and cartilaginous mass in the buccal cavity of many
molluscs, it supports the radula.
Oligomery Division of the body into three linear regions, characteristic of many
deuterostome animals. Tricoelomate.
Oncomiracidium (pl. Oncomiracidia) The ciliated larva of monogeneans.
Oncosphere Encapsulated first stage in the life cycle of certain tapeworms that
bears six hooks and cilia. Typically referred to as a coracidium when released into
the water.
Ontogeny The development of the individual from fertilization to death.
Oostegite A large medial platelike process of a thoracic coxae that contributes to a
marsupium.
Ootype Small, centrally-positioned sac within the female reproductive system of
most parasitic platyhelminths.
Oozooid The zooid developing from the fertilized egg of urochordates.
Operculum (pl. Opercula) A lid or covering of an opening or chamber.
Ophiopluteus (pl. Ophioplutei) Planktotrophic larva of many species of
ophiuroids.
Opisthodetic In bivalves; the hinge ligament situated posterior to the umbo.
Opisthognathus Posteriorly directed position of insect head.
Opisthosoma The posterior end of the pogonophore body which is composed of
numerous (up to 95) segments. The posterior chelicerate tagma, also called the
abdomen.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 24 of 10
Oral arm One of the four, often frilly extensions of the scyphozoan manubrium.
Oral ciliature Cilia that are associated with the mouth region of ciliates.
Oral disc Area around the mouth of an anthozoan polyp which bears eight to
several hundred hollow tentacles.
Oral shield One of a series of large plates that frame the ophiuroid mouth and also
form a chewing apparatus with five triangular, interradial jaws at the center.
Oral sucker Organ that surrounds the trematode mouth, prevents dislodgement
and aids in feeding.
Organ of Tömösvary Hygroreceptive or chemoreceptive organs on the tracheate
head.
Origin One of the two attached ends of a muscle. Of the two, the origin is usually
proximal and remains stationary when the muscle contracts.
Osculum (pl. Oscula) The excurrent opening of the water circulation system of the
sponge.
Osmoconformation Internal osmolarity is allowed to vary with external
osmolarity.
Osmoregulation The maintenance of an internal osmolarity unlike the external.
Ossicle An internal skeletal piece, commonly calcareous as in echinoderms.
Ostium (pl. Ostia) A small incurrent opening or pore on the surface of a body, gill,
or heart.
Ovigerous Bearing eggs externally.
Ovigerous leg The third appendage of pycnogonids, used by the male to brood the
fertilized eggs.
Oviparous Egg-laying.
Paedogenesis Achievement of sexual maturity as a larva, without attaining adult
morphology.
Pallial line The line of mantle attachment impressed on the inner surface of the
shell as a scar.
Pallium Mantle.
Palmella Nonflagellated stage of flagellated protozoans.
Papula (pl. Papulae) Finger-like, respiratory evagination of the aboral body wall
of some asteroids.
Paramylon Photosynthetic storage product of euglenoids.
Paraphyletic A taxon containing some, but not all, of the descendants of an
ancestor.
Parapodium (pl. Parapodia)··Lateral, fleshy, paddle-like appendage on polychaete
annelids.
Parasitism A symbiotic relationship in which one species (parasite) benefits from
the relationship and the other species (host) is harmed but usually not killed.
Parasitoidism A prolonged intimate symbiosis in which one member eventually
kills the other.
Paratomy The phenomenon of linear budding in some turbellarians and annelids.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 25 of 10
Parenchyma Connective tissue compartment between the body wall musculature
and gut of platyhelminths.
Parenchymula A sponge larva that lacks an internal cavity and bears flagellated
cells over all of its outer surface except, often, the posterior pole. Parenchymella.
Parthenogenesis Unisexual reproduction with unfertilized eggs and no
contribution by males.
Parturial molt The molt that results in the appearance of complete, functional
oöstegites.
Patch reef A small circular or irregular reef that rises from the floor of a lagoon
behind a barrier reef or within an atoll.
Paurometabolous Insect development in which nymphs closely resemble adults
but lack wings and are sexually immature. There is no radical metamorphosis.
Paxilla (pl. Paxillae) An echinoderm ossicle crowned with small, movable spines.
Pectinate Having teeth or side branches arranged like a comb.
Pectine A comb-like sensory appendages unique to scorpions.
Pedal disc In some sea anemones, a flattened disc at the aboral end of the column
for attachment.
Pedal laceration Method of asexual reproduction in some anemones in which
parts of the pedal disc are left behind as the animal moves.
Pedicellaria (pl. Pedicellariae) A small, specialized jawlike appendage of asteroids
and echinoids which is used for protection and feeding.
Pedicle Muscular, flexible stalk that attaches articulate brachiopods to the
substratum.
Pedipalp The second chelicerate appendage, it is modified for a variety of
functions.
Peduncle Muscular, flexible attachment stalk of goose barnacles.
Pelagic The water of the open ocean, including the neritic and oceanic zones. Also,
organisms living in the water independent of the bottom.
Pelagosphera Secondary planktotrophic larva of sipunculans.
Pellicle Protozoan “body wall” composed of cell membrane, cytoskeleton, and
other organelles.
Pellucid Clear, transparent.
Penetration anchor An anchor that holds one part of a burrowing animal’s body
in place as another part penetrates and advances into the sediment.
Peniculus (pl. Peniculi) A modified membranelle that is greatly lengthened and
thus tends to be similar to an undulating membrane in function.
Pentactula Metamorphosing stage of holothuroid development that bears five
primary tentacles.
Pentamerous Divided into five parts, characteristic of the body of echinoderms.
Periostracum The outer proteinaceous layer of a molluscan shell, composed
conchiolin.
Periproct The membranous area, often bearing ossicles, around the anus of
echinoids.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 26 of 10
Perisarc A supporting, nonliving chitinous cuticle secreted by the epidermis
surrounding most hydroids.
Peristalsis A wave of muscular contraction moving along a body or internal tube
or vessel. Peristome Buccal cavity of ciliates. The membranous area around the
mouth of some echinoderms, i.e., sea urchins.
Peristomium The first true segment, immediately posterior to the prostomium, of
an annelid. Usually lacks locomotory appendages.
Peritoneum The innermost, noncontractile layer of a stratified coelomic
epithelium; separates the coelomic fluid from the musculature.
Petaloid One of five petal-shaped areas on the aboral surface of irregular urchins
that bear specialized respiratory podia.
Phagocytosis The engulfment of large particles, such as bacteria and protozoans,
by evagination of the cell surface.
Pharynx (pl. Pharynges) An anterior gut region, often heavily muscularized.
Phorozooid A locomotory zooid of doliolids that has a short posterior spur upon
which buds differentiate into gonozooids.
Photocyte Specialized cell within which light is produced.
Photophore A light-producing organ.
Photosynthate The organic carbon fixed by the photosynthetic pathway.
Phyllobranch Having flat, leaflike gills.
Phyllode Each of five oral ambulacral areas of irregular echinoids that contains
specialized podia for obtaining food particles.
Phyllopod Flattened, leaflike appendage.
Phytoflagellate A photosynthetic flagellate.
Phytophagous Plant eating.
Phytoplankton Microscopic algae suspended in the water column of lakes and
seas.
Pilidium A free-swimming and planktotrophic larva of many heteronemerteans
which is characterized by an apical tuft of cilia and is somewhat helmet-shaped.
Pinacocyte One of the epithelial-like flattened cells which together make up the
sponge pinacoderm.
Pinnate Having side branches, like a feather.
Pinnule Side branch of an appendage, i.e., on octocoral tentacles, crinoid arms.
Pinocytosis A nonspecific form of endocytosis in which the rate of uptake is in
simple proportion to the external concentration of the material being absorbed.
Planispiral All whorls of a coiled molluscan shell lying in a single plane.
Plankton Organisms suspended in the water column and unable to move
independently of water current because of small size or insufficient motility.
Planktotrophic larva A planktonic larva that feeds on other planktonic organisms.
Planula (pl. Planulae) A cnidarian larva that is elongated and radially symmetrical
but with anterior and posterior ends.
Plasmodium (pl. Plasmodia) Amoeboid syncytial mass.
Pleopod The anterior abdominal appendages of malacostracans.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 27 of 10
Plerocercoid The final stage in the life cycle of certain tapeworms.
Plesiomorphic Refers to an evolutionarily-primitive state of a homolog.
Pleurite (pl. Pleura) Either of the two primary, lateral, exoskeletal plates of each
segment of an arthropod; also pleuron.
Plicate Folded or ridged.
Podocyst A foot extension of some pulmonate embryos for excretion and
absorption.
Podocyte Cell with branching interdigitating toelike processes, usually over the
surface of a blood vessel. An adaptation for ultrafiltration.
Polyembryony Development of multiple embryos from a single cell mass.
Polymorphism Two or more individuals or zooids of a species modified for
different functions.
Polyp Form of cnidarian that has a thin layer of mesoglea and is generally sessile.
Polyphyletic A taxon that includes the descendants of more than one ancestor.
Polypide The innermost parts of a bryozoan zooid, including the introvert,
lophophore, and viscera but not the body wall or zooecium.
Polypide regression Degeneration and replacement of bryozoan polypide from the
cystid.
Porocyte A sponge cell that surrounds an opening which extends from the external
surface to the spongocoel.
Preoral pit The developmental precursor of the wheel organ and Hatschek’s
groove that opens on the left side of the head of larval cephalochordates.
Pressure drag The difference in pressure at the front end (higher pressure) of a
forward-moving organism as compared to the rear end (lower pressure).
Pretrochal region Apical plate, prototroch, and mouth region of a trochophore
larva.
Primary host See definitive host.
Proboscis (pl. Proboscides) Any tubular process of the head or anterior part of the
gut, usually used in feeding and often extensible.
Proboscis apparatus The complex, eversible, prey-capturing organ of nemerteans.
Proboscis pore The opening of the proboscis apparatus at or near the anterior tip
of a nemertean.
Procercoid Developmental stage of certain tapeworms between oncosphere and
plerocercoid.
Proctodeum Invaginated embryonic ectoderm joining the anus with the
endodermal midgut. Procuticle Thick, inner layer of the arthropod exoskeleton.
Proglottid One of the linearly arranged segment-like sections that make up the
strobila of a tapeworm.
Prognathus Anteriorly directed position of insect head.
Prograde Propagating in the direction in which the animal is moving, ie posterior
to anterior (= direct propagation).
Pronate Rotation of the leading edge down.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 28 of 10
Propodium (pl. Propodia) The front of a gastropod foot which acts like a plough
and anchor.
Prosopyle Internal opening of a sponge through which water flows from the
incurrent canal into a radial canal or flagellated chambers.
Protandry Type of hermaphroditism in which the individual is first a male and
then a female.
Protoconch The shell of the veliger which may remain at the apex of the adult
shell.
Protogyny Type of consecutive hermaphroditism in which the individual is first
female then male.
Protonephridium (pl. Protonephridia) A ciliated excretory tubule capped
internally by one or more terminal cells specialized for ultrafiltration.
Protopod The basal part of a crustacean appendage, consisting of the combined
coxa and basis.
Protostome Member of a major branch of the Animal Kingdom, in which the
blastopore contributes to the formation of the mouth.
Prototroch Preoral ring of cilia of a trochophore larva.
Protozoea Third larval stage of a decapod (shrimp); after the metanauplius and
before the zoea.
Proximal Close to the origin, center, or midline.
Pseudocoel Fluid-filled body cavity that occupies the connective tissue
compartment. Differs from the hemocoel only in the absence of a heart.
Pseudofeces In filter feeders such as bivalves, material removed from the water
flow, aggregated, and rejected before entering the gut.
Pseudolamellibranch gill Bivalve gill with filaments bound together with small
tissue junctions.
Pseudopodium (pl. Pseudopodia) A flowing extension of a cell.
Ptychocyst A cnida that discharges a thread used to weave a tube.
Pygidium (pl. Pygidia) The terminal, nonsegmental part of the body of a
segmented animal. Typically bears the anus. Telson.
Pyriform Pear-shaped.
Pupa (pl. Pupae) In holometabolous insects, the stage between the last larval instar
and the adult.
Pyramid Large calcareous plate that composes Aristotle’s lantern; shaped
somewhat like an arrowhead with the point projected toward the mouth.
Racemose Formed of a number of coalescing ducts draining to a central cavity or
duct.
Radial canal One of five fluid-filled channels of the echinoderm water-vascular
system that join the ring canal to the lateral canals.
Radial cleavage Type of cleavage pattern in which the cleavage spindles or
cleavage planes are at right angles or parallel to the polar axis of the egg.
Radial symmetry The arrangement of similar parts around a central axis.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 29 of 10
Radiole Each of the several pinnate tentacles on the head of a sabellid, serpulid, or
spirorbid polychaete.
Radula (pl. Radulae) A belt of transverse rows of teeth supported by the
odontophore.
Radula sac Pocket of the buccal cavity from which the molluscan radula arises.
Ramus A branch.
Raptorial Animals that capture prey.
Recent The current epoch of the Quaternary Period.
Redia (pl. Rediae Stage in the digenean life cycle between the sporocyst and
cercaria.
Regulated compartment A space, such as an organelle, gut region, or body
cavity, in which the chemical environment can be controlled.
Regulative development Embryonic fate determination in which cell fates are
determined by a network of cellular communication in the embryo.
Relictual A remnant of a once more widespread distribution.
Repugnatorial gland Arthropod gland producing repellent and toxic compounds
for defense.
Reserve stylet One of several accessory reserve stylets present on each side of the
nemertean central proboscis stylet.
Resilium The inner portion of the hinge ligament.
Respiratory tree One of two respiratory organs of most holothuroid echinoderms.
Consists of a network of thin-walled tubules in the perivisceral coelom that
originates from the cloacal wall.
Reticulopodium (pl. Reticulopodia)··A pseudopodium that forms a threadlike
branched mesh and contains axial microtubules.
Retractor muscle Muscle that withdraws an eversible or protrusible body part.
Retrograde Passing in a direction opposite the direction of motion of the animal,
ie anterior to posterior.
Retroperitoneal Outside, or behind, the peritoneum, i.e. outside the coelom but
typically bulging into the coelom and covered by peritoneum.
Rhabdite Platyhelminth epidermal secretion droplets which are characterized
microscopically by a specific, layered ultrastructure.
Rhagon Developmental stage immediately following the metamorphosis of a
demosponge larva. Typically, it is asconoid or syconoid in structure.
Rhinophore One of the second pair of sensory tentacles.
Rhombogen A dicyemid rhombozoan similar to a nematogen but whose axial cell
is in the process of forming an infusoriform larva. A sexually reproductive
nematogen.
Rhopalium (pl. Rhopalia) A club-shaped, marginal sensory organ of scyphozoans.
Rhopalial lappet One of two small, specialized flaps on a rhopalium.
Rhynchocoel A fluid-filled coelomic cavity that houses the retracted nemertean
proboscis.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 30 of 10
Rhynchodeum In nemerteans, the short anterior canal that joins the proboscis pore
to the proboscis.
Ring canal Part of the echinoderm water-vascular system that joins the stone canal
to the radial canals. The marginal canal of the gastrovascular system of some
medusae.
Rostroconchida An extinct class of molluscs that may have been ancestral to
modern bivalves.
Rostrum Middorsal projection in some rotifers that bears cilia and sensory bristles
at its tip and is also adhesive. Median, anteriorly-directed spine from the carapace
and head of some crustaceans.
Saccate nephridium Excretory organ derived from a coelomic end sac and
metanephridial tubule.
Sanguivorous Feeding on blood (= hematophagous).
Saltatory Jumping, leaping locomotion.
Scaphognathite Paddle-like projection of the second maxilla that produces a
ventilating current; gill bailer.
Schizocoel Coelomic cavity derived from the separation, or splitting apart, of a
solid mass of mesodermal cells.
Schizogamy Apicomplexa. Multiple fission that produces merozoites.
Sclerite Thickened, tanned area of cuticle in the exoskeleton of arthropods.
Scleroseptum (pl. Sclerosepta)··One of the many radiating calcareous partitions in
the skeletal cup of stony corals.
Sclerotized Highly tanned (hardened), darkened, and thickened arthropod
exoskeleton.
Scolex (pl. Scoleces Anterior head region of tapeworms that is adapted for
adhering to the host.
Scutum (pl. Scuta) One of the calcareous plates forming the barnacle operculum.
Scyphistoma (pl. Scyphistomae) A scyphozoan polyp.
Segmentation Body composed of a linear series of repeating units, or segments;
metamerism.
Sediment Particles (clay, sand, detritus) deposited on the ocean or lake bottom.
Selective deposit feeding Feeding in which animals selectively remove organic
detritus particles from the surrounding sand particles.
Seminal receptacle Chamber in the female gonoduct for the reception and storage
of allosperm.
Seminal vesicle Part of the male gonoduct that functions in the storage of
autosperm.
Sensillum (pl. Sensilla) Arthropod sense organ involving a specialized part of the
exoskeleton.
Sensu lato (s.l.) In the broad sense.
Sensu stricto (s.s.) In the strict sense.
Septum (pl. Septa) A double-walled tissue partition in the cross-sectional plane of
a bilaterian or a radial plane of a cnidarian.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 31 of 10
Septal filament The free edge of an anthozoan septum that is trilobed.
Sessile In anatomy: attached directly to the body surface and not stalked, also flush
with the body surface . In ecology: attached firmly to a substratum and not free to
move.
Seta (pl. Setae) An exoskeletal bristle composed of a -chitin
Setiger A segment with setae.
Setose Searing setae.
Sexual dimorphism Male and female of a species with different shapes or
appearances.
Shell A rigid skeleton on the outside of an organism. The calcified covering of
molluscs and brachiopods.
Shield A small calcareous plate in certain echinoderms, especially ophiuroids.
Sieve tracheae Arthropod tracheal system in which the spiracle opens into an
atrial or tubelike chamber from which a great bundle of tracheae arises.
Sigmoid S-shaped
Siliceous Composed of silica.
Simple epithelium Composed of a single layer of cells, ie monolayered.
Sinus Saclike space.
Siphon An accessory gut channel of echiurans and some echinoids. A tubular fold
of the molluscan mantle used to direct water to or from the mantle cavity. Inhalant
and exhalant apertures of urochordates.
Siphonoglyph Ciliated groove in the pharyngeal wall of some anthozoans that
moves water into the coelenteron.
Siphonozooid A highly-modified pennatulacean polyp that pumps water into, or
allows it to escape from, the interconnected gastrovascular cavities of the colony.
Siphuncle A strand of tissue in a delicate calcareous tube functions in filling
chambers with gas.
Slug Opisthobranch or pulmonate in which the shell is absent or reduced and
buried in the mantle.
Solenocyte A protonephridial terminal cell with one flagellum and a long
microvillar collar.
Somatic Pertaining to the body.
Somite A body segment or metamere.
Spasmin Ciliate contractile protein which requires ATP for extension.
Speciose Having many species.
Spermatheca (pl. Spermathecae) Another term for a seminal receptacle.
Spheridium (pl. Spheridia) An echinoid statocyst.
Spicule A small needle-like or rodlike skeletal piece.
Spinneret Spinning organ of spiders.
Spiracle Slitlike external opening of the arthropod tracheal system.
Spiral cleavage Type of cleavage pattern in which the cleavage spindles or
cleavage planes are oblique to the polar axis of the egg.
Spire All the whorls of a gastropod shell above the body whorl.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 32 of 10
Spirocyst Cnida with a long adhesive thread that functions in capture of prey and
in attachment to a substratum.
Spongin A large, collagenous, connective tissue fiber of sponges.
Spongiome System of small vesicles or tubules that surrounds the contractile
vacuole in the contractile vacuole complex of ciliates.
Spongocoel Interior cavity of asconoid sponges. Atrium.
Sporocyst Nonciliated second stage in the life cycle of digeneans. Arises from a
miracidium and gives rise to rediae.
Sporosac Incomplete gonophore (made up of only the gonadal tissue) that remains
attached to the polypoid colony.
Sporozoite Apicomplexa. Infective sporelike stage that results from meiosis of the
zygote.
Spring tides Tides occurring on new and full moons characterized by large tidal
amplitude.
Spur A long, slender dorsal appendage of doliolids that trails behind the oozooid
and bears buds. Cadophore.
Squamous epithelium Epithelium of flattened tile-like cells.
Statocyst A sense organ that can provide orientation to the pull of gravity.
Typically composed of a chamber containing concretions (statoliths) in contact
with receptor cells.
Stenohaline Restricted to a narrow range of environmental salinities.
Stenopod A narrow, cylindrical, leglike appendage.
Stereoblastula A solid blastula, lacking an internal cavity or blastocoel.
Stereogastrula A solid gastrula, lacking an archenteron cavity.
Sternite The ventral plate of the cuticle of each segment of an arthropod.
Sternum (pl. Sterna) The combined sternites.
Stolon Rootlike extension of the body that interconnects colonial zooids.
Stomodeum Invaginated embryonic ectoderm joining the mouth with the
endodermal midgut.
Stone canal Part of the echinoderm water-vascular system that joins the
madreporite with the ring canal. Usually, but not always calcified.
Storage excretion Internal, indefinite retention of some excretory products, such
as uric acid.
Stratified epithelium Composed of two or more layers of cells, only one of which
rests on the basal lamina.
Streptoneury Gastropod nervous system twisted by torsion into an asymmetrical
figure-8.
Stridulate To generate sound by rubbing body parts together.
Stridulate To produce sound by rubbing one body part against another.
Strobila (pl. Strobilae) A scyphozoan polyp that buds medusae; or the posterior
part of a tapeworm that consists of proglottids.
Strobilation Process by which scyphomedusae arise as buds that are released by
transverse fission of the oral end of the scyphistoma.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 33 of 10
Stylet A dagger-like structure associated with various systems of different animal
groups.
Stygobiotic Living in caves.
Subchelate A pincer in which the movable finger closes against a flat palm.
Sublittoral The sea floor between the low tide line and the seaward edge of the
continental shelf. Subradula organ Cushion-shaped chemosensory structure of
chitons.
Subumbrella Lower oral surface of a medusa.
Subterminal Located some distance from the end.
Subtidal The sea below the low tide line.
Supratidal Above the high tide line.
Sulcus A longitudinal groove of dinoflagellates that bears the posteriorly directed
flagellum.
Suppinate Rotate the leading edge of a limb up.
Suspension feeding Feeding on organic particles (plankton and detritus)
suspended in water.
Suture The junction between the septum and the wall of a cephalopod shell.
Syconoid sponge A radially symmetrical sponge that has a body wall folded into
radially oriented canals.
Symbiosis An intimate, long-term, physical interraction between two species, in
which at least one of the species is dependent, to various degrees, upon the other.
Symmetrogenic Producing mirror-image daughter cells as a result of fission.
Synanthropic Living with humans.
Syncytium Tissue in which nuclei are not separated by cell membranes.
Synkaryon Zygotic nucleus of ciliates.
Systole The contraction phase of a heart beat.
Tagma (pl. Tagmata) An arthropod body region of arthropods (i.e., head, thorax,
abdomen).
Tanned Stabilization of the arthropod exocuticle by the formation of cross
linkages.
Tapetum A reflective layer within an eye.
Tan To increase the strength, and darken the color, of protein by establishing
crosslinks between adjacent polypeptides.
Tarsal organ Cuplike spider chemoreceptor for detecting pheromones.
Taxodont Hinge dentition and consisting of uniform alternating teeth and sockets
in a row.
Taxon A group of organisms with a common ancestor.
Tegmen Membranous oral wall of the crinoid disc.
Tegument The nonciliated outer syncytial layer of the body wall of parasitic
platyhelminths and acanthocephalans.
Telolecithal Type of egg in which the yolk material is concentrated to one side
(vegetal) of the egg.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 34 of 10
Telopodite The movable part of an appendage extending outward from an
immovable protopod. Telotroch A ring of cilia encircling the anus at the posterior
end of a trochophore larva.
Tensilium The outer portion of a bivalve hinge ligament.
Tentacle Evagination of the body wall surrounding the mouth which aids in the
capture and ingestion of food.
Tentacle sheath In Bryozoa, the part of the withdrawn body wall that encloses the
withdrawn tentacles of the lophophore. See vestibula.
Tergite The dorsal, sclerite of each arthropod segment.
Tergum (pl. Terga) The combined tergites. A plate contributing to the barnacle
operculum.
Terminal At the end.
Terminal anchor Anchor at the leading end of a burrowing animal.
Terminal cell Tubular flagellated cell attached to the inner end of the
protonephridial tubule.
Test An encasing or shell-like skeleton, typically covered externally by cytoplasm
or living tissue.
Theca (pl. Thecae)··The nonliving cuticle around the hydranths of thecate
hydroids. Hydrotheca.
Thecate Refers to hydroids with a hydrotheca surrounding the polyp proper.
Tetramerous Radial symmetry in which a basic pattern is repeated in multiples of
four.
Thigmotactic Responding to touch or surface contact.
Thoracopod Any thoracic appendage of an arthropod.
Tiedemann’s body One of the interradial outpockets of the ring canal of many
echinoderms. Removes unwanted particulates from the water-vascular system.
Tongue bar A downgrowth of pharyngeal tissue that divides a developing gill
opening into two side-by-side slits.
Tornaria Transparent, long-lived, planktotrophic larva of enteropneusts.
Torsion The counterclockwise twist of the gastropod visceral mass over the head
and foot.
Toxicyst A vesicular organelle in the pellicle of gymnostome ciliates which
discharges long threads with bulbous bases; used for defense or capturing prey.
Transmitted light In microscopy, light, from a source below the stage, which
passes through the plane of the stage to reach and pass through the object.
Trichobranchiate Having filamentous gills.
Trichocyst A bottle-shaped extrusible organelle of the ciliate pellicle.
Trilobite larva Horseshoe crab larva that superficially resembles trilobites.
Triploblastic Embryos possessing all three germ layers: ectoderm, endoderm, and
mesoderm.
Triturate To grind or masticate.
Trochophore Type of larva found in molluscs, annelids, and other groups in
which the larval body is ringed by a girdle of cilia, the prototroch.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 35 of 10
Trochus The anterior band of cilia of the divided corona of some rotifers.
Troglobitic, troglodytic Dwelling in caves or otherwise underground.
Trophi Cuticular hard parts of the rotifer mastax.
Trophosome Central mass of tissue in the trunk of the pogonophore that is packed
with symbiotic bacteria.
Trophozoite Apicomplexa. Feeding stage that occurs when the sporozoite invades
the host.
Trophozooid Nutritive or feeding zooid of doliolid urochordates.
Tropic hormone A hormone whose target is an endocrine cell.
Tube tracheae Simple branched or unbranched trachea.
Tubicolous Tube-dwelling.
Tubules of Cuvier Eversible toxic or sticky tubules associated with the bases of
the respiratory trees of some holothuoid echinoderms.
Tubulus (pl. Tubuli) Sensory papilla on the trunk of some aschelminths.
Tunic Special cuticular covering of the body of ascidians.
Tunicate A urochordate.
Tunicin A kind of cellulose that forms structural fibers in ascidian tunics.
Typhlosole A ridge projecting internally from the intestinal wall to increase its
surface area.
Ultrafiltration Passage of fluid across a fine-mesh filter to retain proteins and
larger particles.
Umbo (pl. Umbos, Umbones) A dorsal protuberance of a bivalve valve rising
above the hinge.
Uncinus (pl. Uncini) A minute seta modified into a hook.
Undulating membrane Type of ciliary organelle that is a row of adhering cilia
forming a sheet.
Uniramous Having one branch.
Ureotelic Producing urea as the end product of nitrogen metabolism.
Uricotelic Producing uric acid as the end product of nitrogen metabolism.
Uropods Sixth abdominal appendages of most malacostracans but the 4 th, 5 th, and
6 th of amphipods.
Vanadocytes Yellowish-green ascidian blood cells that contain high
concentrations of vanadium.
Vascular plug Specialized nemertean exchange site across which an ultrafiltrate
passes from the blood to the rhynchocoel.
Vegetative nucleus Macronucleus.
Velarium Velum-like structure of cubozoans.
Veliger Planktotrophic molluscan larva that follows the trochophore.
Velum Shelf formed by the margin of the umbrella projected inward which is
characteristic of most hydromedusae. One of the two ciliated flaps with which a
veliger larva swims and feeds.
Vermiform Having the shape of a worm.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 36 of 10
Vermiform embryo Asexually-produced young of dicyemids that has the same
form as the parent; formed within the axial cell of the parent.
Vessel A small tubular blood channel.
Vestibule Preoral chamber. In Bryozoa, a space enclosed by the withdrawn body
wall of a retracted zooid distal to the withdrawn tentacles and tentacle sheath.
Vestigial Reduced to a non-functional remnant.
Vestimentum The collar-like body region of a vestimentiferan that helps to secrete
the animal’s tube.
Vibraculum (pl. Vibracula Bristle-like heterozooid found in some cheilostome
bryozoans.
Visceral mass One of three primary parts of the molluscan body; contains the
internal organs.
Viscous drag Friction that results from the tendency of the polar water molecules
to stick to each other and to surfaces.
Vitellarium (pl. Vitellaria) Specialized part of the ovary for the production of
yolk-filled nurse cells. Nonfeeding barrel-shaped larval stage of some
echinoderms.
Viviparous Embryos gestated internally within the female where supplemental
nutrition is supplied.
Whorl Any complete turn (360 ° ) of a coiled molluscan shell.
Xylophagous Feeding on wood.
Zoarium (pl. Zoaria) The form of a bryozoan colony.
Zoea (pl. Zoeae) Penultimate larval stage of many decapod crustaceans, preceding
the postlarva.
Zoochlorella (pl. Zoochlorellae) Unicellular green algal symbiont of certain
animals, especially freshwater sponges and freshwater and marine cnidarians and
turbellarians.
Zooecium (pl. Zooecia) The cuticle, or exoskeleton, of a bryozoan zooid.
Zooflagellate A flagellate that has one to many flagella, lacks chloroplasts, and is
heterotrophic.
Zooplankton Microscopic animals suspended in the water of oceans and
freshwater lakes.
Zooplanktivore Feeding on zooplankton.
Zooxanthella (pl. Zooxanthellae) A golden-brown alga, usually a dinoflagellate,
symbiotic with various marine animals, especially cnidarians.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 37 of 10
2 LECTURE
Leture #1. Animal kingdom. Subkingdom - celled animals Protozoa. Species
diversity and structural features of Sarkomastigofora.
1. Simple as a whole organism. Similarity in the structure of cells and the
simplest multicellular animals. General characteristics of subkingdoms.
2. Type Sarcomastigophora. Organelles of Sarkomastigofora movement.
Sexual process.
3. Modern taxonomy of Sarkomastigofora.
1. Simple as a whole organism. Similarity in the structure of cells and the
simplest multicellular animals. General characteristics of subkingdoms.
The protozoa are a heterogeneous assemblage of some 50,000 single-cell
organisms possessing typical (eukaryote) membrane-bound cellular organelles.
Because most are motile and many are heterotrophic, this assemblage was treated
in the past as a single phylum within the Animal Kingdom— the phylum Protozoa.
They are now known to consist of a number of different unicellular phyla, which
together with most algal phyla are placed in the Kingdom Protista. Some of these
protozoan groups are related to each other, some probably evolved independently
from remote eukaryote ancestors, and some are members of various algal groups.
The unicellular level of organization is the only characteristic by which the
protozoa as a whole can be described; in all other respects they display extreme
diversity. Protozoa exhibit all types of symmetry, a great range of structural
complexity, and adaptations for all types of environmental conditions. As
organisms, the protozoa have remained at the unicellular level but have evolved
along numerous lines through the specialization of parts of the protoplasm
(organelles) or of the skeletal structure. Thus, simplicity and complexity in
protozoa are reflected in the number and nature of their organelles and skeletons in
the same way that simplicity and complexity in multicellular animals can be
reflected in the development of tissues and organ system s. A protozoan cell may
be far more complex than a metazoan cell, but a protozoan cell is an entire
organism , not part of an organism, as is a metazoan cell.
Protozoa occur wherever moisture is present—in the sea, in all types of fresh
water, and in the soil. There are commensal, mutualistic, and many parasitic
species.
Although most protozoa occur as solitary individuals, there are numerous
colonial forms. Some colonial forms, such as species of Volvox, attain such a
degree of cellular interdependence that they approach a true multicellular level of
structure (Fig. 2-6). Both solitary and colonial species may be either free moving
or sessile.
Protozoan Organelles and General Physiology
The protozoan body is usually bounded only by the cell membrane, which
possesses the typical bilayered lipid ultrastructure of cells in general. The rigidity
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 38 of 10
or flexibility of the protozoan body is largely dependent on the nature of the
underlying cortical cytoplasm, called ectoplasm , which is rather gelatinous,
in contrast to the more fluid, internal cytoplasm called endoplasm. Nonliving
external coverings or shells occur in many different groups. Such coverings may be
sim ple gelatinous or cellulose envelopes, or they may be distinct shells, com
posed
of various inorganic and organic materials, or sometimes foreign particles
cemented together.
Depending on the species, there are one to many nuclei. The locomotors
organelles may be flagella, cilia, or flowing extensions of the body called
pseudopodia. Since the type of locomotors organelle is important in the
classification of the phylum, discussion of the structure of these organelles is
deferred until later.
All types of nutrition occur in protozoa. Some are autotrophic or saprozoic;
many ingest food particles or prey and digest this food intracellular
within food vacuoles. Food reaches the vacuole by engulfment, or phagocytosis,
often through a mouth, or cytostome. Soluble food may enter by pinocytosis.
Intracellular digestion has been most studied in amoebas and ciliates. The food
vacuoles undergo definite changes in hydrogen ion concentration (pH) and in size
during the course of digestion. Following ingestion, the vacuole contents become
increasingly acid and smaller, as excess water is removed. Lysosomes deliver
hydrolytic enzymes (Fig. 2-1), and the vacuole increases in size and becomes
alkaline. The enzymes digest the vacuole contents, and products of digestion then
pass into the cytoplasm by pinocytosis. The indigestible remnants are egested.
Protozoa that live in water where there is active decomposition of organic
matter or in the digestive tract of other animals can exist with little or no oxygen
present. Some protozoa are facultative anaerobes, utilizing oxygen when present
but also capable of anaerobic respiration. Changing availability of food supply and
of oxygen associated with decay typically results in a distinct succession of
populations and protozoan species (see Bick, 1973).
Metabolic wastes diffuse to the outside of the organism. Ammonia is the
principal nitrogenous waste, and the amount eliminated varies directly with the
amount of protein consumed.
Characteristic of many protozoa is an organelle system called the contractile
vacuole complex (Fig. 2-2). The complex is com posed of a spherical vesicle—
the contractile vacuole proper— and a surrounding system of small vesicles or
tubules termed the spongiome. The complex functions primarily in water balance
(osmoregulation), pumping excess water out of the organism . The spongiome
provides for the collection of water, which is delivered to the contractile vacuole.
The latter expels the fluid to the outside of the organism through a temporary or
perm anent pore. In some protozoa (some amoebas and flagellates) the vacuole
completely disappears following contraction and is reformed by fusion of small
vesicles. In others (many ciliates) the vacuole collapses at discharge and is refilled
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 39 of 10
by fluid from the surrounding tubules of the spongiome.
Reproduction and Life Cycles
The protozoan reproductive processes and life cycles are varied. Only a few
of the more common terms are described here.
Asexual reproduction occurs in most protozoa and is the only known m ode of
reproduction in some species. Division of the animal into two or more daughter
cells is called fission. When this process results in two similar daughter cells, it is
termed binary fission; when one daughter cell is much smaller than the other, the
process is called budding. In some protozoa, multiple fission, or schizogony, is the
rule. In schizogony, after a varying number of nuclear divisions, the cell divides
into a number of daughter cells. With few exceptions, asexual reproduction
involves som e replication of missing organelles following fission.
Sexual reproduction may involve fusion (syngamy) of identical gametes
(called isogametes) or gametes that differ in size and structure. The latter, called
anisogametes, range from types that differ only slightly in size to welldifferentiated sperm and eggs. Meiosis commonly occurs in the formation
of gametes, but in many flagellate protozoa and sporozoans meiosis is postzygotic,
that is, it occurs following the formation of the zygote as in most algae (Fig. 2-3).
In ciliate protozoa there is no formation of distinct gametes; instead, two animals
adhere together in a process called conjugation, and they exchange nuclei. Each
migrating nucleus fuses with a stationary nucleus in the opposite conjugant to form
a zygote nucleus (synkaryon). Less com m on is a process called autogamy, in
which two nuclei, each representing a gamete, fuse to form a zygote, all within a
single individual.
Encystment is characteristic of the life cycle of many protozoa, including the
majority of freshwater species. In forming a cyst, the protozoon secretes a
thickened envelope about itself and becomes inactive. Depending on the species,
the protective cyst is resistant to desiccation or low temperatures, and encystment
enables the animal to pass through unfavorable environmental conditions.
The simplest life cycle includes only two phases: an active phase and a protective,
encysted phase. However, the more complex life cycles are often characterized by
encysted zygotes or by for motion of special reproductive cysts, in which fission,
gametogenesis, or other reproductive processes take place.
Protozoa may be dispersed long distances in either the motile or encysted
stages. Water currents, wind, and mud and debris on the bodies of water birds and
other animals are com m on agents of dispersion.
SUM MARY
1 Protozoa are unicellular organism s that are animal-like in being heterotrophic
and motile. In all other respects, protozoa are a very diverse assemblage,
and the major groups are now commonly treated as separate phyla of eukaryote
protistans. The old phylum name Protozoa can be used as a convenient term of
reference for any member of these phyla.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 40 of 10
2 Most protozoa inhabit the sea or fresh water, but there are m any parasitic,
commensal, and mutualistic species.
3 In contrast to metazoans, complexity in protozoa has proceeded through
development and specialization of organelles or skeletal structures. Although
a protozoon is a single cell, it is also a complete organism .
4 Digestion occurs intracellularly within a food vacuole, and food reaches the
vacuole through a cell mouth or by engulfment.
5 Excess water is usually eliminated by a contractile vacuole.
6 Most of the members of the protozoan phyla are distinguished, in part, by their
type of locomotor organelles: flagella, pseudopodia, or cilia.
7 Reproduction by fission occurs at sometime in the life history of almost all
protozoa. Meiosis, gamete formation, and fertilization have been observed in many
species, but the nature of these events and their occurrence in the life cycle of the
organism is highly variable. Encystment is common.
2. Type Sarcomastigophora. Organelles of Sarсomastigophora movement.
Reproduction process. Phylum Sarcomastigophora
These protozoa possess flagella or pseudopodia as locomotor or feeding
organelles and a single type of nucleus. The 18,000 described species constitute the
largest phylum of protozoa, which is composed of two principal groups subphyla),
the flagellates and the sarcodines.
SUBPHYLUM MASTIGOPHORA
The flagellates, or mastigophorans, include those protozoa that possess
flagella as adult organelles. They can be conveniently divided into phytoflagellates
and zooflagellates. The phytoflagellates usually bear one or two flagella and
typically possess chloroplasts. These organism s are thus plantlike, and
phycologists treat most species in this division as algae. The phytoflagellate
division contains most of the free-living members of the class and includes such
common forms as Euglena, Chlamydomonas, Volvox, and Peranema. The
zooflagellates possess one to many flagella, lack chloroplasts, and are either
holozoic or saprozoic. Some are free living, but the majority are commensal,
symbiotic, or parasitic in other animals, particularly arthropods and vertebrates.
Many groups have become highly specialized. It is generally agreed that this
division does not represent a closely related phylogenetic unit.
Locomotion
The presence of flagella is the distinguishing feature of flagellates. The
phytoflagellates usually have one or two flagella, the zooflagellates one to
many. When two or many flagella occur, they may be of equal or unequal length,
and one may be leading and one trailing, as in Peranem a (Fig. 2 - 5 B) and
the dinoflagellates.
The ultrastructures of flagella and cilia are fundamentally similar in all
eukaryote organisms. A single flagellum (or cilium) is constructed very much like
a cable. Two central microtubules form a core that is in turn encircled by nine
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 41 of 10
double outer microtubules (Fig. 2 -4A). One microtubule of each doublet bears two
rows of projections (arms) directed toward the adjacent doublet (Fig. 2-4B). The
entire bundle is enclosed within a sheath that is continuous with the cell
membrane. The flagellum always arises from a basal body, sometimes called
a blepharoplast in flagellates, that lies just beneath the surface. Like a centriole, the
basal body has an ultrastructure somew hat like a flagellum, except that the central
fibrils are absent and the nine fibrils in the outer circle are in triplets, two of the
three being continuous with the doublets of the flagellum. Arms are absent and the
inner microtubule of each triplet is connected by a radial strand to a central ring for
part of its length (Fig. 2-4B).
A fibrillar root system connecting the basal body with various organelles,
especially the nucleus, characterizes m any flagellates. In some species the basal
body functions as a centriole in mitosis.
Flagellar propulsion in most astigophorans essentially follow s the same
principle as that of a propeller, the flagellum undergoing undulations in one or two
planes that either push or pull. The undulatory waves pass from base to tip and
drive the organism in the opposite direction (Fig. 2-4C ), or the undulations pass
from tip to base and pull the organism (Fig. 2-4D ). In many species with two
flagella, the actual path of movement is determined by the flagellar orientation.
Other types of beat have been described for flagella besides undulatory.
The relationship of flagellar (or ciliary) ultrastructure to movement has received
much attention, and the sliding tubule model is now widely accepted. According to
this theory, the microtubules do not change length but adjacent doublets slide past
each other, causing the entire organelle to bend. Sliding involves the establishment
of cross bridges and utilization of adenosine triphosphate (ATP), as in muscle
contraction (see Sleigh, 1974).
Mastigophorans that have thin, flexible pellicles are often capable of am
eboid m ovem ent; some forms, such as chrysomonads, may cast off their flagella
and assume an ameboid type of locomotion entirely.
Nutrition
Phytoflagellates are primarily autotrophic and contain chlorophyll. When the
chlorophyll is not masked by other pigments, a flagellate appears green in color,
like the phytom onads and euglenids. If the xanthophylls dominate, the color is red,
orange, yellow, or brown.
Strict heterotrophic nutrition occurs in zooflagellates as well as some other groups,
and there are many parasitic species. The mechanism s of food capture and
ingestion vary greatly, and the methods employed by some of the better known
groups will be described in the following sections Phytoflagellates store reserve
foods, such as oils or fats, or they may store carbohydrates as typical plant starch
or in other forms. In zooflagellates, glycogen is the usual reserve food product.
SUM MAR Y
1 Flagellates are distinguished by the presence of one or more flagella.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 42 of 10
2 Classically, the group has included many autotrophic groups (phytoflagellates),
such as chrysomonads, euglenids, and the volvocids. They possess chlorophyll
plus other pigm ents and store such food materials as oils, fats, and starches (other
than glycogen). These groups are more properly assigned to the various algal
phyla.
3 The remaining heterotrophs (zooflagellates) are a small, heterogeneous
assemblage. A few are free living, but most are parasitic, commensal, or
mutualistic in other animals.
4 Flagella (and cilia) are composed of microtubules surrounded by the plasma
membrane. The arrangement of m icrotubules in which nine pairs (doublets)
surround two central m icrotubules is with few exceptions characteristic of flagella
and cilia in all eukaryote organism s. Movement of the organelle is thought to
result from the sliding ofated with the microtubules relative to each other. Each
flagellum (or cilium] arises from a basal body, or kinetosome.
5 Flagella commonly beat by undulation in two planes. The beat pushes or pulls
the flagellate, and the path of movement depends on the point of flagellum
attachment and the combined action, when there is more than one flagellum.
SUBPHYLUM SARCODINA
The subphylum Sarcodina contains those protozoa in which adults possess
flowing extensions of the body called pseudopodia. Pseudopodia are used for
capturing prey in all Sarcodina, and in benthic groups, pseudopodia are also used
as locom otor organelles.
The subphylum includes the familiar amebas as well as many other marine,
freshwater, and terrestrial forms. The slime molds are sometimes included in the
Sarcodina, but in the following discussion, the slim e m olds will be considered
to be fungi and left to the mycologists.
The Sarcodina either are asymmetrical or have a spherical symmetry. They
possess relatively few organelles and in this respect are perhaps the simplest
protozoa. However, skeletal structures, which are found in the majority of species,
reach a complexity and beauty that is surpassed by few other organisms.
The presence of flagellated gametes among many Sarcodina and the tendency of
many flagellates to lose their flagella during some phase of the life cycle, often
becoming ameboid, are important reasons for uniting the mastigophorans and
sarcodines within a single phylum. These facts would also seem to indicate that the
Mastigophora are the ancestral group.
SUM M ARY
1. Members of the phylum Sarcodina are distinguished by the presence of flowing
extensions of cytoplasm called pseudopodia, which are used in feeding and, in
some, for locomotion. The pseupseudopodia are given different names, depending
on their shape and structure.
2 Although organelles have remained relatively simple, many Sarcodina have
evolved complex skeletons. The various classes of Sarcodina are distinguished
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 43 of 10
by the nature of their skeletons and their pseudopodia.
3 The marine, freshwater, and parasitic naked amebas have no special skeletal
structures and possess large, com m only tubular lobopodia or small, straplike
filopodia, which are used for both feeding and locomotion.
4 Shelled am ebas, which are largely restricted to fresh water, are covered by a
shell composed of secreted organic material or of foreign mineral material
cemented together. A large aperture permits the protrusion of lobopodia or
filopodia.
5 Foraminiferans, which are largely benthic marine Sarcodina, possess a
calcareous test that is usually multicham bered. A single large opening
permits the protrusion of cytoplasm , which may cover the exterior of the test.
Long, delicate, and often anastomosing reticulopodia extend from the protruded
cytoplasm and are used in food trapping and locomotion.
6 Heliozoans are spherical, radially arranged, floating, and benthic Sarcodina that
are largely restricted to fresh water. Long, radiating, needle-like pseudopodia
(axopods) are used in trapping food. The axopods arise from the interior (medulla)
and extend through an outer ectoplasmic cortex, which is com m only vacuolated.
The cortex often contains a siliceous skeleton of plates, tubes, and needles.
7 Radiolarians are marine planktonic Sarcodina with spherical bodies and radiating
axopods. An organic capsule wall separates a central cortex from extracapsular
cytoplasm . Radiolarians have complex skeletons of silicon dioxide or strontium
sulfate within the extracapsular cytoplasm , organized in the form of lattice spheres
or radiating spines or both.
3. Modern taxonomy of Sarcomastigophora.
SYSTEM ATIC RESUME OF SUBPHYLUM MASTIGOPHORA*
One to many flagella present. Asexual reproduction by binary, more or less
symmetrogenic, fission. Autotrophic or heterotrophic or both.
Class Phytomastigophora. Mostly free-living, plantlike flagellates with or
without chromoplasts and usually one or two flagella.
Order Chrysomonadida (Chrysophyta).
Small flagellates with yellow or brown chromoplasts and two unequal flagella.
Siliceous scales commonly cover the body. M arine and freshwater. Chromulina,
Ochromonas, Synura.
Order Silicoflagellida [Chrysophyta).
Flagellum single or absent and chromoplasts brown. Internal siliceous skeleton.
Marine. Dictyocha. Known mostly from fossil forms.
Order Coccolithophorida (Haptophyta).
Tiny marine flagellates covered by calcareous platelets— coccoliths. Two flagella
and yellow to brown chromoplasts. No endogenous siliceous cysts. Coccolithus,
Rhabdosphaera.
Order Heterochlorida [Xanthophyta).
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 44 of 10
Two unequal flagella and yellow-green chromoplasts. Siliceous cysts.
Heterochloris, Myxochloris.
Order Cryptomonadida [Cryptophyta],
Compressed, biflagcllate, with an anterior depression or reservoir. Two
chromoplastids, usually yellow to brown or colorless. Marine and freshwater.
Chilomonas is a com m on colorless genus in polluted water.
Order Dinoflagellida [Pyrrophyta].
An equatorial and a posterior longitudinal flagellum located in grooves. Body
either naked or covered by cellulose plates or valves or by a cellulose membrane.
Brown or yellow chromoplasts and stigma usually present, but there are many
colorless species. Largely marine; some parasites. Includes the marine genera
Gonyaulax, N octiluca, Histiophysis, and Ornithocercus, and the marine and
freshwater genera Glenodinium , Gymnodinium,Ceratium , O odinium , and
Symbiodinium.
Order Ebriida.
Biflagellate, with no chromoplasts; internal siliceous skeleton. Mainly fossil.
Ebria.
Order Euglenida (Euglenophyta).
Elongated green or colorless flagellates with two flagella arising from an anterior
recess. Stigma present in colored forms. Primarily freshwater. Euglena, Phacus,
Peranema, Rhabdomonas.
‘ Order Chloromonadida (Chloromonadophyta or Rhaphidiophyta]
Small, dorsoventrally flattened flagellates with numerous green chromoplasts. Two
flagella, one trailing. Gonyostom um .
Order Volvocida (Chlorophyta; order Volvocales).
Body with green, usually single, cup-shaped chromoplast, stigma, and often two to
four apical flagella per cell. Some colorless forms. Many colonial species. Largely
freshwater forms. Chlamydomonas,Polytomella,Haematococcus, Gonium,
Pandorina, Platydorina, Eudorina, Pleodorina, Volvox.
Class Zoomastigophora.
Flagellates with neither chromoplasts nor leucoplasts. One to many flagella, in
most cases with basal granule complex. Many commensals, symbionts, and
parasites.
Order Choanoflagellida.
Freshwater flagellates, with a single flagellum surrounded by a collar. Sessile,
sometimes stalked, sometimes with lorica; solitary or colonial. Codosiga,
Proterospongia, Salpingoeca.
Order Rhizomastigida.
Ameboid forms, with one to many flagella. Chiefly freshwater. M astigamoeba,
Dimorpha.
Order Kinetoplastida.
One or two flagella emerging from a pit. Mostly parasitic. Bodo, Leishmania,
Trypanosoma.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 45 of 10
Order Retortamonadida.
Gut parasites of insects or vertebrates, with two or four flagella. One flagellum
associated ventrally located cytostome. Chilomastix.
Order Diplomonadida.
Bilaterally symmetrical flagellates, with one or two nuclei,each nucleus associated
with one to four flagella. Mostly parasites. Hexamita,Giardia.
Order Oxymonadida.
Commensal or mutualistic flagellates in the guts of insects; a few in vertebrates.
One to many nuclei, each nucleus associated with four flagella, some of which are
turned posteriorly and adhere to body surface. Oxymonas, Pyrsonympha.
Order Trichomonadida.
Parasitic flagellates. Four to six flagella, one of which is trailing. Trichomonas
(Fig. 2 -9A).
Order Hypermastigida.
Many flagella, with kinetosomes arranged in a circle, plate, or longitudinal or
spiral rows. Symbionts in guts of termites, cockroaches, and wood roaches.
Lophomonas, Trichonym pha, Barbulanympha.
Superclass Opalinata.
Body covered by longitudinal, oblique rows of cilia rising from anterior
subterminal rows. Two or many monomorphic nuclei. Binary fission generally
symmetrogenic. Sexual reproduction involves syngamy with flagellated
gametes. Gut commensals of anurans; less commonly of fishes, salamanders, and
reptiles. O palina, Zelleriella.
SYSTEM ATIC RESUME OF SUBPHYLUM SARCODINA
Protozoa with pseudopodia as feeding and locomotor organelles; flagella, when
present, only in developmental stages. Little development of cortical organelles.
Skeletons of various form s and composition characteristic of some groups.
Superclass Rhizopoda.
Lobopodia, filopodia, or reticulopodia used for locomotion and feeding.
Class Lobosa.
Pseudopodia, usually lobopods.
Subclass Gymnamoeba.
Amebas that lack shells.
Order Amoebida.
Naked amebas that lack flagellated stages. Largely freshwater, some marine; many
parasites. Chaos,Amoeba, Entameoba, Hydramoeba.
Order Schizopyrenida.
Naked amebas with flagellated stages. Marine, freshwater, and soil species.
Naegleria, Acantham oeba.
Order Pelobiontida.
Naked, multinucleated amebas with one pseudopod and no flagellated stages.
Pelomyxa.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 46 of 10
Subclass Testacealobosa.
Amebas with shells.
Order Arcellinida, or Testacida.
Body enclosed in a shell or test with an aperture through which the pseudopodia
protrude. Free living, largely in fresh water. A rcella, Difflugia, Centropyxis.
Class Filosa.
Amebas with filopods.
Order Aconchulinida.
Naked amebas. Freshwater and parasites of algae. Vampyrella.
Order Testaceafilosida.
Shelled amebas. Mostly in fresh water and soil. Gromia,Euglypha.
Class Granuloreticulosa.
Sarcodina with delicate granular reticulopodia.
Order Foraminiferida.
Chiefly marine species with mostly multichambered shells. Shells may be organic,
but most commonly are calcareous. Globigerina, Orbulina, D iscorbis, Spirillina,
Nummulites.
Superclass Actinopoda.
Primarily floating or sessile Sarcodina with actinopodia radiating from a spherical
body.
Class Acantharia.
Radiolarians with a radiating skeleton of strontium sulfate; axopodia. Most without
a central capsule separating endoplasm and ectoplasm . Marine. Acanthom etra.
Class Polycystina.
Radiolarians with a siliceous skeleton and a perforated capsular membrane.
Thalassicola, Collozoum , Sphaerozoum.
Class Phaeodaria.
Radiolarians with a siliceous skeleton but a capsular membrane containing
only three pores. Aulacantha.
Class Heliozoa.
Without central capsule. Naked, or if skeleton present, of siliceous scales and
spines. Primarily in fresh water. Actinophrys, A ctinosphaerium , Camptonem a.
Lecture #2. Features of the life cycle and structure of Sporozoa.
1. Features of the organization due to the parasitic way of life. Structure apical
complex cells. Alternation of asexual and sexual reproduction.
2. General characteristics Class spore.
3. Sporozoa lifecycle.
1. Features of the organization due to the parasitic way of life. Structure
apical complex cells. Alternation of asexual and sexual reproduction
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 47 of 10
The Sporozoans: Phyla Apicomplexa and Microspora
Sporozoans are parasitic protozoa, living within or between cells of their
invertebrate or vertebrate hosts. They belong to two phyla, the Apicomplexa and
the Microspora, both form erly composing an old protozoan grouping, the
Sporozoa. Sporozoan, which refers to the presence of sporelike stages, continues to
be used as a com m on name.
Most known sporozoans and all those of known economic and medical
importance belong to the phylum Apicomplexa, so named because of a complex
of ringlike, tubular, filamentous organelles at the apical end, visible only with the
electron m icroscope (Fig. 2-21). The function of the apical complex is uncertain
but may include entry into the host cell. One or more feeding pores are located on
the side of the body.
Figure 2-21 Lateral view of a generalized aptcomplexan sporozoan. (From Farmer,
J. N., 1980: The Protozoa. С. V. Mosby Co., St. Louis, p. 360.)
SYSTEMATIC RESUME OF THE SPOROZOANS
PHYLUM APICOMPLEXA
With apical complex at some stage. Spores usually present but lacking polar
filaments. All species parasitic.
Class Sporozoa.
Reproduction sexual and asexual.
Subclass Gregarinia.
Mature trophozoites large and occur in host's gut and body cavities. Parasites of
annelids and arthropods. Gregarina, Monocystis (common parasite of earthworm's
seminal receptacles).
Subclass Coccidia. Mature trophozoites small and intracellular. Eimeria, Isospora,
Aggregata, Plasmodium, Toxoplasma.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 48 of 10
Class Piroplasmea.
Parasites of vertebrate red blood cells transmitted by ticks. No spores. Theileria,
Babesia.
PHYLUM MICROSPORA
Spores with polar filament present. All species parasitic. Nosema.
2. General characteristics Class spore.
SUMMARY
1 Sporozoans are parasitic protozoa belonging to two phyla, the Sporozoa and the
Microspora. Some species possess sporelike infective stages, from which the name
sporozoan is derived.
2 The phylum Apicomplexa contains the gregarines, which are parasites of insects
and ane-lids, and the coccidians, which are intracellular parasites of gut and blood
cells of vertebrates and invertebrates. Plasmodium, the causal agent of malaria, is
the best known and most familiar coccidian.
3 The complex life cycles usually involve fission (schizogony), sexual
reproduction (gamogony), and spore formation (sporogony).
4 The phylum Microspora contains intracellular parasites, especially of insects.
The name microspora is derived from the spore, which contains filaments that can
be everted.
3. Sporozoa lifecycle.
The life cycle of apicomplcxans typically involves an asexual and a sexual
phase (Fig. 2-22). An infective stage, called a sporozoite, invades the host and
undergoes asexual m ultiplication by fission, producing individuals called
merozoites. Merozoites can continue schizogamy but eventually form gametes
(gamogony) that fuse to form a zygote. The zygote undergoes meiosis to form
sporozoites.
The nature and life cycle of apicomplexan sporozoans can be illustrated by
the coccidians, which include the parasites that cause malaria in humans. Malaria
continues to be one of the worst scourges of mankind. About 300 million people
are believed to be infected each year. The untreated disease can be long lasting and
terribly debilitating. Malaria has played a m ajor and often unrecognized role in
human history. The name means literally "bad air" because the disease was
originally thought to be caused by the air of swamps and marshes. Although
malaria had been recognized since ancient times, the causative agent was not
discovered until 1880, when a physician with the French army in North Africa
identified the coccidian parasite Plasmodium in the blood cells of a malarial
patient. In 1887 the mosquito was recognized to be the vector.
The introduction of the parasite into a human host is brought about by the
bite of certain species of mosquitoes, which inject the sporozoites along with their
salivary secretions into the capillaries of the skin (Fig. 2-23). The parasite is
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 49 of 10
carried by the bloodstream to the liver, where it invades a liver cell. Here further
development results in asexual reproduction through multiple fission. These
daughter cells invade other liver cells and continue to reproduce. After a week or
so there is an invasion of red blood cells by parasites produced in the liver. Within
the red cell the parasite increases in size and undergoes multiple fission. The
individuals (merozoites) produced by fission within the red cells escape and invade
other red cells. The liberation and reinvasion are not continuous but occur
simultaneously from all infected red blood cells. The timing of the event depends
on the period of time required to complete the developmental cycle within the
host's cells. The release causes chills and fever, the typical symptom s of malaria.
Eventually, some of the parasites invading red cells do not undergo fission but
become transformed into gametocytes. The gametocyte remains within the red
blood cell. If such a cell is ingested by a mosquito, the gametocyte is liberated
within the new host's gut. After some further development, a male gametocyte
(microgametocyte) fuses with a fem ale gametocyte (macrogametocyte) to
form a zygote. The zygote penetrates the stomach wall and gives rise to a large
number of spore stages (sporozoites). It is these stages, which migrate to the
salivary glands, that are introduced into the hum an host by the bite of the
mosquito.
The asexual stage of other coccidians occurs in blood cells or in gut cells. A
number of diseases of domesticated animals are caused by coccidians. The genus
Eimeria, for example, affects chickens, turkeys, pigs, sheep, and cattle (Fig. 2-22).
Another common group of apicomplexans contains the gregarines, which attain the
largest size among the sporozoans. They are parasites of invertebrates, especially
annelids and insects, and therefore not of economic importance. Intracellular parasitic species are only a few microns long, but those that inhabit the body or gut
cavities of the host may reach 10 mm in length. The body of a gregarine
trophozoite is elongate (Fig. 2-24), and the anterior part sometimes possesses
hooks, a sucker or suckers, or a simple filament or knob for anchoring the parasite
into the host's cells. The host becomes infected through ingesting spores containing
sporozoites of the parasites (Fig. 2-25). Depending on the species, the liberated
gregarine sporozoites either remain in the gut of the host or penetrate the gut wall
to reach other areas of the body. The life cycle commonly lacks schizogony.
Members of the class Piroplasmea are another small group of sporozoans that also
attack the red blood cells of vertebrates. Spores and gametes are not produced, and
the parasites are transmitted by ticks. Pathogenic infections in cattle and other domesticated animals are of considerable economic importance.
The phylum Microspora contains a smaller number of intracellular parasites, but
they are found in most animal groups, especially arthropods. They lack the apical
complex of other sporozoans, and the sporelike stage is characterized by a polar
filament that is extruded when this stage is taken into the host. The filament
appears to be involved in some way with the invasion of the host's cell. As
parasites of the honeybee and silkworm, the microsporidians are of economic
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 50 of 10
importance. One of the early studies of sporozoans was that of Pasteur in 1870, on
Nosema bombycis in silkworms.
Figure 2-22 Life cycle of an eimcriid coccidian, a destructive intracellular parasite
of the gut epithelium of many vertebrates, including domesticated buds and
mammals.
EMCD 042-18-35.1.31/ 03-2014
SEXUAL CYCLE
TISSUE CYCLE
IN RED BLOOD CELL
IN LIVER CELL
(gametocytes)
(cryptozoites)
#2 edition from 16.06. 2014
p. 51 of 10
ASEXUAL CYCLE
IN RED BLOOD CELL
(merozoites)
Figure 2-23 The life cycles of Plasmodium in a mosquito and in man. Reinvasion
of liver cells in the tissue cycle does not occur in Plasmodium falciparum.
Figure 2-24 Trophozoites of the gregarinc Gregarina gamhami attacking the
midgut epithelium of a locust.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 52 of 10
Figure 2-25 Life cycle of a gregarine, Stylocephalus longicollis, an intestinal
parasite of a beetle. There is no schizogony in this species.
Lecture # 3. Species diversity and structural features of ciliates.
1. General characteristics of ciliates as the most highly differentiated and
protozoa.
2. Reproduction of ciliates (conjugation).
3. The main classes of ciliates.
1. General characteristics of ciliates as the most highly differentiated and
protozoa.
Phylum Ciliophora
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 53 of 10
The phylum Ciliophora is the largest and the most homogeneous of the principal
protozoan groups, and all evidence indicates that they share a common
evolutionary ancestry (Fig. 2-43). Some 7200 species have been described, and
many groups are still not well known.
All possess cilia or compound ciliary structures as locomotor or foodacquiring organelles at some time in the life cycle. Also present is an infraciliary
system, composed of ciliary basal bodies, or kinetosomes, below the level of the
cell surface and associated with fibrils that run in various directions. Such an
infraciliary system may be present at all stages in the life cycle even with marked
reduction in surface ciliation. Most ciliates possess a cell mouth, or cytostome. In
contrast to the other protozoan classes, ciliates are characterized by the presence of
two types of nuclei: one vegetative (the macronuclcus, concerned with the
synthesis of RNA as well as DNA) and the other reproductive (the micronucleus,
concerned only with the synthesis of DNA). Fission is transverse, and sexual reproduction never involves the formation of free gametes.
Ciliates are widely distributed in both fresh and marine waters and in the water
films of soil. About one third of ciliate species are ecto- and endocom-mensals or
parasites.
Form and Structure
The body shape is usually constant and in general is asymmetrical; however, radial
symmetry with an anterior mouth is probably the primitive condition (Fig. 2-26).
Although the majority of ciliates are solitary and free swimming, there are both
sessile and colonial forms. The bodies of tintinnids and some heterotrichs,
peritrichs, and suctorians are housed within a lorica, a girdle-like encasement,
which is either secreted or composed of foreign material cemented together. In the
peritrichs the lorica is attached to the substratum, but in many others the lorica is
carried about by the organism (Fig. 2-27).
Figure 2-26 Prorodon, a primitive ciliate. (After Faure-Fremict from Corliss.)
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 54 of 10
Figure 2-27 Tintinnopsis, a marine ciliate with lorica, or test, composed of foreign
particles. Note conspicuous membranelles and tentacle-like organelles interspersed
between them. (After Faure-Fremiet from Corliss.)
The ciliate body is typically covered by a complex, living pellicle, usually
containing a number of different organelles. The pellicular system has been studied
in detail in numerous genera, including Paramecium. There is an outer limiting
plasma membrane, which is continuous with the membrane, surrounding the cilia.
Beneath the outer membrane are closely packed vesicles, or alveoli, which are
moderately to greatly flattened (Figs. 2-28 and 2-29). The outer and inner
membranes bounding a flattened alveolus would thus form a middle and inner
membrane of the ciliate pellicle. Between adjacent alveoli emerge the cilia and
mucigenic or other bodies (Fig. 2-29). Beneath the alveoli are located the
infraciliary system, the kinetosomes, and fibrils. The alveoli contribute to the
stability of the pellicle and perhaps limit the permeability of the cell surface
(Pitelka, 1970).
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 55 of 10
Figure 2-28 Section through cilium and pellicle of Colpidium. Note that alveoli are
greatly flattened and their inner and outer membranes fused at base of cilium. At
top right is an enlarged view of surface and alveolar membranes. At lower right is
a cross section of a cilium and surrounding pellicle taken at the level indicated by
the dashed line. Note the circle of nine doubled peripheral ciliary fibrils. (After
Pitelka.)
Figure 2-29 Pellicular system in Paramecium. (After Ehret and Powers from
Corliss.)
The pellicle of the familiar Paramecium has inflated kidney-shaped alveoli
(Fig. 2-29). The inflated condition and the shape of the alveoli produce a polygonal
space about the one or two cilia that arise between them. Alternating with the alveoli are bottle-shaped organelles, the trichocysts, which form a second, deeper,
compact layer of the pellicular system.
The trichocyst is a peculiar rodlike organelle characteristic of many ciliates that
can be explosively discharged as a filament. In the undischarged state they are
oriented at right angles to the body surface The discharged trichocyst consists of a
long, striated, threadlike shaft surmounted by a barb, which is shaped somewhat
like a golf tee (Fig. 2-30). The shaft is not evident in the undischarged state and is
probably polymerized in the process of discharge. The function of trichocysts is
uncertain, but they may be used in anchoring the ciliate when feeding.
Toxicysts are vesicular organelles found in the pellicle of gymnostomes (Dileptus
and Didinium), which on discharge consist of bulbous bases that taper into long
threads (Fig. 2-31A and B). Toxicysts are used for defense or for capturing prey by
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 56 of 10
paralysis and cytolysis. They are commonly restricted to the parts of the ciliate
body that contact prey, such as around the smooth region in Didinium.
Figure 2-30 Electron micrograph of discharged trichocysts of Paramecium. Note
golf-tee-shaped barb and long, striated shaft. (By Jacus and Hall, 1946: Biol. Bull.,
91:141.)
outer ciliary membrane
Mucigenic bodies (mucocysts) are another group of pellicular organelles found in
some ciliates. They are arranged in rows like trichocysts and discharge a mucoid
material that may function in the formation of cysts or protective coverings (Fig. 228).
Cilia have the same structure as flagella; they differ from flagella chiefly in
that they are generally more numerous and shorter. Compound ciliary organelles,
evolved from the adhesion of varying numbers of individual cilia, are of common
occurrence and will be described later.
The ciliature can be conveniently divided into the body (or somatic)
ciliature, which occurs over the general body surface, and the oral ciliature, which
is associated with the mouth region. Distribution of body cilia is quite variable. In
the primitive groups, cilia cover the entire animal and are arranged in longitudinal
rows (Fig. 2-26), but in many of the more specialized groups they have become
limited to certain regions of the body.
As mentioned earlier, each cilium arises from a basal body or kinctosome,
located in the alveolar layer (Figs. 2-29). The kinetosomcs that form a particular
longitudinal row arc connected by means of fine, striated fibrils, called kinetodesma. The cilia, kinetosomcs, and fibrils of a row make up a kinety. The
longitudinal bundle of fibrils runs to the right side of the row of kineto-somes, and
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 57 of 10
each kinctosome gives rise to one ki-netodesmos (fibril), which joins the
longitudinal bundle and extends anteriorly. Single kinctodes-mata arc tapered and
extend for varying distances as parts of the bundle. At the kinctosome, the kinctodesmata are connected to certain of the kinctosome triplets.
Figure 2-33 Reconstruction of section of the pellicle of Tetrahymena. Right side is
on viewer's left. Abbreviations: kinetodesmos |k); transverse microtubules (tm);
postciliary microtubules (pm); longitudinal microtubules (lm); basal microtubules
(bm); alveolus (a); cilium (c); epiplasm (c); mitochondrion |m); mucigenic body
(mb). (From Allen, R. D, 1967: Fine structure, reconstruction and possible function
of components of the cortex of Tetrahymena pyriformis. J. Protozool., 14:553565.)
In addition to kinetodesmata, there are also ribbons of microtubules that
extend posteriorly and transversally from the kinetosome and apparently function
as part of the cilium anchorage system.
A kinety system is characteristic of all ciliates, although there are variations
in details of the pattern. Even groups such as the Suctorida, which possess cilia
only during developmental stages, retain part of the kinety system in the adult.
Locomotion
The ciliates are the fastest moving of the protozoa. In its beat each cilium
performs an effective and a recovery stroke. During the effective stroke the cilium
is outstretched and moves from a forward to a backward position (Fig. 2-34A and
B). In the recovery stroke the cilium is bent over to the right against the body
(when viewed from above and looking anteriorly) and is brought back to the forward position in a counterclockwise movement. The recovery position offers less
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 58 of 10
water resistance and is somewhat analogous to feathering an oar. A cilium moves
in three different planes in the course of a complete cycle of beat, and the positions
have been captured and recorded in scanning electron micrographs of freeze-dried
Paramecium (Tamm, 1972).
Figure 2-33 Reconstruction of section of the pellicle of Tetrahymena. Right side is
on viewer's left. Abbreviations: kinetodesmos |k); transverse microtubules (tm);
postciliary microtubules (pm); longitudinal microtubules (lm); basal microtubules
(bm); alveolus (a); cilium (c); epiplasm (c); mitochondrion |m); mucigenic body
(mb). (From Allen, R. D, 1967: Fine structure, reconstruction and possible function
of components of the cortex of Tetrahymena pyriformis. J. Protozool., 14:553565.)
58 The Protozoa
In addition to kinetodesmata, there are also ribbons of microtubules that extend
posteriorly and transversally from the kinetosome and apparently function as part
of the cilium anchorage system.
A kincty system is characteristic of all ciliates, although there are variations in
details of the pattern. Even groups such as the Suctorida, which possess cilia only
during developmental stages, retain part of the kinety system in the adult.
Locomotion
The ciliates are the fastest moving of the protozoa. In its beat each cilium performs
an effective and a recovery stroke. During the effective stroke the cilium is
outstretched and moves from a forward to a backward position (Fig. 2-34A and B).
In the recovery stroke the cilium is bent over to the right against the body (when
viewed from above and looking anteriorly) and is brought back to the forward
position in a counterclockwise movement. The recovery position offers less water
resistance and is somewhat analogous to feathering an oar. A cilium moves in three
different planes in the course of a complete cycle of beat, and the positions have
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 59 of 10
been captured and recorded in scanning electron micrographs of freeze-dried
Paramecium (Tamm, 1972).
Figure 2-34 Ciliary beating and locomotion. A, Cycle of a ciliary beat seen from
the side. In the effective stroke the Cilium is outstretched and moves from left to
right. B, Path described by tip of cilium during beat cycle as seen from surface. E
is effective stroke and R is the recovery stroke. (From Sleigh, M. A., 1973: The
Biology of Protozoa. Edward Arnold Publishers, London, p. 38.) C, Series of
adjacent cilia in a metachronal wave in various stages of the beat cycle. Direction
of effective stroke (solid arrow) is same as metachronal wave (dashed arrow).
Letter с indicates wave crest. D, As in В except metachronal wave is moving in
opposite direction to effective stroke. |C and D from Jones, A. R., 1974: The
Ciliates. St. Martin's Press, Hutchinson in London, p. 70.) E, Metachronal waves in
Paramecium during forward swimming. Wave crests are shown by lines and their
direction by arrows (dotted on opposite side). Movement of cihate is indicated by
large arrow. (From Machemer, H., 1974: Ciliary activity and metachronism in
Protozoa. In Sleigh, M. A. (Ed): Cilia and Flagella. Academic Press, London, p.
224.) F, The avoiding reaction of Paramecium. (After Hyman, L. H., 1940: The
Invertebrates, McGraw-Hill Book Co., N.Y. Vol. I.)
The movements of adjacent cilia are coupled as a result of interference
effects of the surrounding water layers. Thus, hydrodynamic forces impose a
coordination on the cilia. The beat of individual cilia, rather than being random or
synchronous, is part of the metachronal waves that sweep along the length of the
body (Fig. 2-34C). Most commonly, the metachronal waves pass at right angles to
the beat stroke, but there are variations in the pattern (see Sleigh, 1973). There is
no evidence that the in-fraciliature of fibrils functions as a conducting system in
coordination. They may serve primarily in anchorage.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 60 of 10
In forms such as Paramecium the direction of the effective stroke is oblique
to the long axis of the body (Fig. 2-34E). This causes the ciliate to swim in a spiral
course and at the same time to rotate on its longitudinal axis. The ciliary beat can
be reversed, and the animal can move backward. This backward movement is
associated with the so-called avoiding reaction. In Paramecium, tor example, when
the animal comes in contact with some undesirable substance or object, the ciliary
beat is reversed (Fig. 2-34F). The animal moves backward a short distance, turns
slightly clockwise or counterclockwise, and moves forward again. If unfavorable
conditions are still encountered, the avoiding reaction is repeated. External stimuli
are probably detected through certain long, stiff cilia that play no role in movement
and are probably entirely sensory. The direction and intensity of the beat are
controlled by levels of Ca++ and К/ ions (Eckert, 1972).
The highly specialized hypotrichs, such as Urostyla, Stylonychia, and
Euplotes (Fig. 2-32Л), have greatly modified body cilia. The body has become
differentiated into distinct dorsal and ventral surfaces, and cilia have largely
disappeared except on certain areas of the ventral surface. Here the cilia occur as a
number of tufts, called cirri. The cilia of a cirrus beat together, and coordination
here is believed to result from some sort of structural coupling as a result of the
close proximity of their bases.
Some ciliates, especially sessile forms, can undergo contractile movements,
either shortening the stalk by which the body is attached, as in Vorticella (Fig. 232C), or shortening the entire body, as in Stentor (Fig. 2-32D). Contraction is
brought about by bundles of contractile filaments, or myonemes, that lie in the
pellicle. In Vorticella and the colonial Carchesium, both of which have bell-shaped
bodies attached by a long slender stalk, the myonemes extend into the stalk as a
single, large, spiral fiber. The contractions of this spiral my-oneme, which
functions very much like a coiled spring, produce the familiar popping movements
that are so characteristic of Vorticella and related genera.
Nutrition
Feeding in ciliates parallels, on a microscopic level, feeding in multicellular
animals. Typically a distinct mouth, or cytostome, is present, although it has been
secondarily lost in some groups. In primitive groups the mouth is located anteriorly
(Fig. 2-26), but in most ciliates it has been displaced posteriorly to varying
degrees. The mouth opens into a canal or passageway called the cytopharynx,
which is separated from the endoplasm by a membrane. It is this membrane that
enlarges and pinches off as a food vacuole. The wall of the cytopharynx is
strengthened with rods (nemades-mata) arranged like the staves of a barrel. Primitively, the ingestive organelles consist only of the cytostome and cytopharynx
(Figs. 2-26 and 2-35Л), but in the majority of ciliates the cytostome is preceded by
a preoral chamber. The preoral chamber may take the form of a vestibule, which
varies from a slight depression to a deep funnel, with the cytostome at its base
(Fig. 2-35B). The vestibule is clothed with simple cilia derived from the somatic
ciliature.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 61 of 10
In the higher ciliates the preoral chamber is typically a buccal cavity, which
differs from a vestibule by containing compound ciliary organelles instead of
simple cilia (Fig. 2-35C to F). There are two basic types of such ciliary organelles:
the undulating membrane and the membranelle. An undulating membrane is a row
of adhering cilia forming a sheet (Fig. 2-36Л and B). A membranelle is derived
from two or three short rows of cilia, all of which adhere to form a more or less
triangular or fan-shaped plate and typically occur in a series (Figs. 2-27, 2-32D,
and 2-36B). Although there is no actual fusion of adjacent cilia in these compound
organelles, their kinetosomes and bases are sufficiently close together to produce
some sort of structural coupling that causes all of the cilia of a membranelle to beat
together.
The term peristome, which is commonly encountered in the literature, is
synonymous with buccal cavity. In members of a number of orders the buccal
organelles project from the buccal cavity, or, as in the Hypotrichida (Fig. 2-32A),
the buccal cavity is somewhat shallow so that the organelles occupy a flattened
area around the oral region. Such an area is called the peristomial field. In forms
like Paramecium there is a vestibule in front of the buccal cavity (Figs. 2-35D and
2-36D).
Figure 2-35 Oral areas of various ciliates. A, In rhabdophorine gymnostomes, such
as Coleps, Prorodon, and Didi-nium. B, In a trichostome such as Colpoda, with a
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 62 of 10
vestibule that is displaced from anterior end. C, In a tetrahymenine hymenostome,
such as Tetrahymena. D, In a peniculine hymenostome, such as Paramecium. E, In
a peritrich, such as Vorticella. F, In a hypotrich, such as Euplotes. (Modified after
Corliss, J. O., 1961: The Ciliated Protozoa. Pergamon Press, N.Y.)
The free-swimming holozoic species display several types of feeding habits.
Some are raptorial, and attack and devour rotifers, gastrotrichs, protozoans, and
other ciliates. A smaller number, including Nassula, are herbivorous on algae and
diatoms. Many have become specialized for suspension feeding. The oral
apparatus of raptorial ciliates is typically limited to the cytostome and
cytopharynx.
Didinium has perhaps been most studied of all the raptorial feeders. This
little barrel-shaped ciliate feeds on other ciliates, particularly Paramecium (Fig. 237A). When Didinium attacks a Paramecium, it discharges toxicysts into the
Paramecium and the proboscis-like anterior end attaches to the prey through the
terminal mouth, which can open almost as wide as the diameter of the body.
An interesting group of raptorial ciliates is the aberrant subclass Suctoria, formerly
considered a separate class. Free-living suctorians are all sessile and are attached to
the substratum directly or by means of a stalk (Fig. 2-37). Cilia are present only in
the immature stages. The body bears tentacles, which may be knobbed at the tip or
shaped like long spines (Fig. 2-41B). Each tentacle is supported by a cylinder of
microtubules and carries special organelles, called haptocysts (Fig. 2-37D).
Suctorians feed on other ciliates, and when prey strikes the tentacles, the
haptocysts are discharged into the prey body, anchoring it to the tentacles (Figs. 237D to F and 2-38). The contents of the prey are then sucked through the tubular
tentacle into the suctorian, where they are collected into food vacuoles.
Typically characteristic of suspension feeders is the buccal cavity. Food for
suspension feeders consists of any small organic particles, dead or living,
particularly bacteria that are suspended in water. Food is brought to the body and
into the buccal cavity by the compound ciliary organelles. From the buccal cavity
the food particles are driven through the cytostome and into the cytopharynx.
When the particles reach the cytopharynx, they collect within a food vacuole.
The order Hymenostomatida—"membrane-mouthed"—contains some of the most
primitive suspension feeders. Tetrahymena is a good example of such a primitive
type (Fig. 2-36B). The cy tostome is located a little behind the leading edge of the
body. Just within the broad opening to the buccal cavity are four ciliary
organelles—an undulating membrane on the right side of the chamber and three
membranellcs on the left. The three membranelles constitute an adoral zone of
mcm-branelles, which in many higher groups of ciliates is much more developed
and extensive.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 63 of 10
Figure 2-36 A, Pleuronema. (After Noland from Corliss.) B, Tetrahymena. (After
Corliss, J. O., 1961: The Ciliated Protozoa. Pergamon Press, N.Y.) C, Scanning
electron photomicrograph of Uronychia, a marine ciliate, showing the highly
developed membranelles. (By Small, E. В., and Marszalek, D. S., 1969: Science,
163: 1064-1065. Copyright 1969, American Association for the Advancement of
Science.) D, Buccal organelles of Paramecium. (After Yusa from Man-well.) £,
Lacrymaria. (After Conn from Hyman.)
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 64 of 10
j. \ в
Figure 2-37 A, Four Didinium, raptorial ciliates, attacking one Paramecium. (After
Mast from Dogiel.| B, Acineta, a suctorian. (After Calkins from Hyman.| C-F,
Suctorian haptocysts and prey capture. Haptocyst isolated |C| and within tentacle
tip (D). Attachment of tentacle to prey (£) and en-gulfmcnt through tentacle (PL
(From Sleigh, M. A., 1973: The Biology of Protozoa, Edward Arnold Publishers,
London, p. 64. Based on micrographs of Rudzinska, Bar D E F dele, and Grell.J
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 65 of 10
Figure 2-38 A colony of the suctonan Heliophrya feeding on Paramecium. Some
individuals of Paramecium have just been captured. Others have been ingested to
various degrees. (From Spoon et al., 1976: Observations on the behavior and
feeding mechanisms of the suctorian Heliophrya erhardi preying on Paramecium.
Trans. Am. Micros Soc 95-443-462.|
In Paramecium, the most familiar genus of the order, an oral groove along
the side of the body leads posteriorly to a vestibule, located about midway back
from the anterior end. The vestibule, buccal cavity, and cytopharynx together form
a large, curved funnel (Figs. 2-35D and 2-36D). The undulating membrane, here
called the endoral membrane, runs transversely along the right wall and marks the
junction of the vestibule and buccal cavity. The three membranelles are also
modified. Two, called peniculi, arc greatly lengthened and thus tend to be more
similar to an undulating membrane in function than to the more typical
membranelle.
In feeding, the cilia of the oral groove produce a current of water that sweeps
in an arclike manner down the side of the body and over the oral region. The
ciliature of the vestibule and buccal cavity pull in food particles and drive them
into the forming food vacuole.
In the subclass Peritricha, whose members possess little or no somatic cilia,
the buccal ciliary organelles are highly developed and form a large, disclike,
peristomial field at the apical end of the animal. In the much-studied peritrich
genus Vor-ticella, a peripheral shelflike projection can close over the disc when the
animal is retracted (Figs. 2-32C and 2-35E). The ciliary organelles lie in a
peristomial groove between the edge of the disc and the peripheral shelf and
consist of two ciliary bands, which wind in a counterclockwise direction around
the margin of the disc and then turn downward into the funnel-shaped buccal
cavity. The inner ciliary band produces the water current and the outer band acts as
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 66 of 10
the filter. Suspended particles, mostly bacteria, are transported along with a stream
of water between the two bands into the buccal cavity.
Ciliates of the subclass Spirotricha, which includes such familiar forms as
Stentor, Halteria, Spi-rostomum, and Euplotes, arc typically suspension feeders.
They usually possess a highly developed adoral zone of many membranelles (Fig.
2-32Л and D).
Within the cytopharynx of all ciliates, food particles enter the food vacuole.
When the food vacuole reaches a certain size, it breaks free from the cytopharynx,
and a new vacuole forms in its place. Detached vacuoles then begin a more or less
circulatory movement through the endoplasm.
Digestion follows the usual pattern, and a pH as low as 1.4 has been reported
during the acid phase-in some species (Paramecium). Following digestion, the
waste-laden food vacuole moves to a definite anal opening, or cytopyge (Fig. 2-1),
at the body surface and expels its contents. The cytopyge varies in position. In
Tetrahymena it is located on the side of the animal, near the posterior end (Fig. 21), whereas in the pcritrichs it opens into the buccal cavity.
There arc relatively few parasitic ciliates, although there arc many ecto- and
endocommensals. Many suctorians are commensals, and a few arc parasites. Hosts
include fishes, mammals, various invertebrates, and other ciliates. Sphaerophyra,
for example, lives within the endoplasm of Stentor, and Endosphaera is parasitic
within the body of the peritrich Telotrochidium.
Other interesting commensal ciliates include Kerona, a little crawling
hypotrich, and Tricho-dina. a mobile peritrich, which are cctocommen-sals on the
surface of hydras. There arc also some free-swimming pcritrichs that occur on the
body surfaces of freshwater planarians, tadpoles, sponges, and other animals.
The genus Ralantidium includes many species that are endocommensals or
endoparasites in the guts of insects and many different vertebrates. Bal-antidium
coli is an endocommensal in the intestines of pigs and is passed by means of cysts
in the feces. This ciliatc has occasionally been found in humans, where in
conjunction with bacteria it erodes pits in the intestinal mucosa and produces
pathogenic symptoms. The related highly specialized Entodiniomorphida (Fig. 239) live as harmless commensals in the digestive tracts of many different hoofed
mammals. Like the flagellate symbionts of termites and roaches, some of them ingest and break down the cellulose of the vegetation eaten by their hosts. The
products of digestion are utilized by the host.
A few ciliates display symbiotic relationships with algae. The most notable
of these is Paramecium hursaria, in which the endoplasm is filled with green
zoochlorellae.
Water Balance
Contractile vacuoles are found in both marine and freshwater species, but
especially in the latter, in which they may discharge as rapidly as every few
seconds. In some species a single vacuole is located near the posterior, but many
species possess more than one vacuole (Fig. 2-31С). In Paramecium one vacuole is
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 67 of 10
located at both the posterior and the anterior of the body (Fig. 2-34F). The
vacuoles are always associated with the innermost region of the ectoplasm and
empty through one or two permanent pores that penetrate the pellicle. The spongiome contains a network of irregular tubules, which may empty into the vacuole
directly or by way of collecting tubules .
When there is more than one vacuole present, they pulsate at different rates
depending on their positions. For example, in Paramecium the posterior vacuole
pulsates faster than the anterior vacuole because of the large amount of water being
delivered into the posterior region by the cytopharynx. Although contractile
vacuoles may be present in marine species, the rate of pulsation is considerably
slower than that in freshwater species; they arc probably removing ingested water.
2. Reproduction of ciliates (conjugation).
Reproduction
Ciliates differ from almost all other organisms in possessing two distinct
types of nuclei—a usually large macronucleus and one or more small micro-nuclei.
The micronuclei arc small, rounded bodies and vary in number from l to as many
as 20, depending on the species. They are diploid, with little RNA. The
micronucleus is a store of genetic material, is responsible for genetic exchange and
nuclear reorganization, and also gives rise to the ma-cronuclei. The macronucleus
is sometimes called the vegetative nucleus, since it is not critical in sexual
reproduction. However, the macronucleus is essential for normal metabolism, for
mitotic division, and for the control of cellular differentiation, and it is responsible
for the genie control of the phenotype through protein synthesis.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 68 of 10
Figure 2-40 Macronuclei (in gray) of various ciliates (micronuclei, in black). A,
Euplotes. B, Vorticella. C, Paramecium. D, Stentor. (After Corliss, J. О., 1961:
The Ciliated Protozoa. Pergamon Press, N.Y.)
One or more macronuclei are present, and they may assume a variety of shapes
(Fig. 2-40). The large macronucleus of Paramecium is somewhat oval or bean
shaped and is located just anterior to the middle of the body. In Stentor and
Spirostomum the macronuclei are long and arranged like a string of beads. Not
infrequently the macronucleus is in the form of a long rod bent in different configurations, such as а С in Euplotes or a horseshoe in Vorticella. The macronucleus is
highly polyploid, the chromosomes having undergone repeated duplication
following the micronuclear origin of the macronucleus. The macronuclei include
numerous nucleoli with RNA.
asexual reproduction
Asexual reproduction is always by means of binary fission, which is
typically transverse. More accurately, fission is described as being homothctogenic, with the division plane cutting across the kinetics—the longitudinal rows of
cilia or basal bodies (Fig. 2-41Л). This is in contrast to the symmetrogenic fission
of flagellates, in which the plane of division (longitudinal) cuts between the rows
of basal granules. Mitotic spindles arc formed only in the division of the
micronuclei. Division of the macronuclei is usually accomplished by constriction.
When a number of macronuclei arc present, they may first combine as a single
body before dividing. The same is true of some forms with beaded or elongated
macronuclei.
Modified fission in the form of budding occurs in some ciliate groups,
notably the Suctoria. In most members of this subclass the parent body buds off a
varying number of daughter cells from the outer surface (Fig. 2-41B); or there is an
internal cavity or brood chamber, and the buds form internally from the chamber
wall. In contrast to the sessile adults, which lack cilia, the daughter cells, or buds,
are provided with several circlets of cilia and are free swimming (Fig. 2-41C).
Following a few hours of free existence, the "larva" attaches and assumes the
characteristics of the sessile adults.
Although there are no centrioles, the kincto-somes of many ciliates, like the
basal granules of flagellates, divide at the time of fission. Furthermore, the
kinetosomes play a primary role in the re-formation of organelles. It has been
found that all of the organelles can be re-formed providing the cell contains a piece
of macronucleus and some kinetosomes. In the more primitive ciliates, in which
the cilia have a general distribution over the body surface, the kinetosomes have
equal potentials in the re-formation of organelles.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 69 of 10
Figure 2-41 A, Homothctogcnic type of fission, in which the plane of division cuts
across the kinetics. [After Corliss.) B, Suctorian Ephelota with external buds.
(After Noble from Hyman.) C, Detached bud of Dendrocometes. (After Pestel
from Hyman.) D, Conjugation in Vorticella. Note the small nonsessilc
microconjugant. (After Kent from Hyman.)
However, in the specialized ciliates there is a corresponding specialization of the
kinetosomes; only certain ones are involved in the re-formation of new cellular
structures during fission. For example, in hypo-trichs such as Euplotes, all of the
organelles are re-sorbed at the time of fission, and certain of the kinetosomes on
the ventral side of the animal divide to form a special group that is organized in a
definite field or pattern. These special "germinal" kinetosomes then migrate to
different parts of the body, where they form all of the surface organelles—cirri,
peristome, cytopharynx, and other structures.
sexual reproduction
An exchange of nuclear material by conjugation is involved in sexual
reproduction. By apparently random contact in the course of swimming, two
sexually compatible members of a particular species adhere in the oral or buccal
region of the body. Following the initial attachment, there is degeneration of
trichocysts and cilia (but not kinetosomes) and a fusion of protoplasm in the region
of contact. Two such fused ciliates are called conjugants; attachment lasts for
several hours. During this period a reorganization and exchange of nuclear material
occurs (Fig. 2-42Л to F). Only the micron-uclci are involved in conjugation; the
macronucleus breaks up and disappears either in the course of or following
micronuclear exchange.
The steps leading to the exchange of micronuclear material between the two
conjugants are fairly constant in all species. After two meiotic divisions of the
micronuclei, all but one of them degenerate. This one then divides, producing two
gametic micronuclei that are genetically identical. One is stationary; the other will
migrate into the opposite conjugant. The migrating, or "male," nucleus in each
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 70 of 10
conjugant moves through the region of fused protoplasm into the opposite member
of the conjugating pair. There the "male" and "female" nuclei fuse with one
another to form a "zygote" nucleus, or synkaryon.
Shortly after nuclear fusion the two animals separate; each is now called an
exconjugant. After separation, there follow in each exconjugant a varying number
of nuclear divisions, leading to the rcconstitution of the normal nuclear condition
characteristic of the species. This reconstitution usually, but not always, involves a
certain number of cytosomal divisions. For example, in some forms where there is
but a single macronucleus and a single micronucleus in the adult, the synkaryon divides once. One of the daughter nuclei forms a micronucleus; the other forms the
macronucleus Thus, the normal nuclear condition is restored without any
cytosomal divisions.
A
b
c
d
e
f
g
Figure 2-42 Sexual reproduction in Paramecium caudatum. A to F, Conjugation. В
to D, Micronuclei undergo three divisions, the first two of which are meiotic. E,
"Male" micronuclei are exchanged. F, They fuse with the stationary micronucleus
of the opposite conjugant. G, Exconjugant with macronucleus and synkaryon
micronucleus; other micronuclei have been resorbed. (Modified after Calkins from
Wichterman.)
However, in Paramecium caudatum, which also possesses a single nucleus
of each type, the synkaryon divides three times, producing eight nuclei. Four
become micronuclei and four become macronuclei. The animal now undergoes two
cytosomal divisions, during the course of which each of the four resulting daughter
cells receives one macronucleus and one micronucleus. In those species that have
numerous nuclei of both types, there is no cytosomal division; the synkaryon
merely divides a sufficient number of times to produce the requisite number of
macronuclei and micronuclei.
In some of the more specialized ciliates, the conjugants are a little smaller
than nonconjugating individuals, or the two members of a conjugating pair are of
strikingly different sizes. Such dioecious macro- and microconjugants occur in
Vorticella (Fig. 2-41D) and represent an adaptation for conjugation in sessile
species. The macroconjugant, or "female," remains attached, while the small bell
of the microconjugant, or "male," breaks free from its stalk and swims about. On
contact with an attached macroconjugant the two bells adhere. A synkaryon forms
only in the macroconjugant from one gametic nucleus contributed by each conjugant. However, there is no separation after conjugation, and the little "male"
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 71 of 10
conjugant degenerates. In the Suctoria conjugation takes place between two
attached individuals that happen to be located side by side.
Lecture #4. Lower tissue. Beam. Type Sponges. Skeletal structure.
Reproduction.
1. Theory of the origin of multicellular organisms.
2. Class calcareous sponge-Calcarea.
3. Class Glass sponges - Hyalospongia.
4. Class Ordinary sponge - Demospongia. The main representatives of classes
of type sponges.
1.Theory of the origin of multicellular organisms
Origin of Metazoa
Most zoologists agree that metazoans have a common ancestry from some
unicellular organism, but there have been differing views as to the particular group
of unicellular forms involved and the mode of origin.
Hadzi (1953) and Hanson (1977) have been the chief proponents of a ciliate
origin for metazoans. Their theory, which may be called the Syncytial Theory,*
holds that multicellular animals arose from a primitive group of multinucleate
ciliates. The ancestral metazoan was at first syncytial in structure but later became
compartmented or cel-lularized when it acquired cell membranes, which produced
a typical multicellular condition. Since many ciliates tend toward bilateral
symmetry, proponents of the Syncytial Theory maintain that the ancestral
metazoan was bilaterally symmetrical and gave rise to the acoel flatworms, which
are therefore held to be the most primitive living metazoans. That the acoels are in
the same size range as the ciliates, are bilaterally symmetrical, are ciliated, and
tend toward a syncytial condition is considered evidence supporting the primitive
position of this group. The ciliate macronucleus, which is absent in acoels, is
assumed to have been absent in the multinucleate protociliate stock from which the
metazoans arose; according to this theory, it developed later in the evolutionary
line leading to the higher ciliates.
There are a number of objections to the Syncytial Theory. Nothing
comparable to cellularization occurs in the ontogeny of any of these groups, t
Furthermore, a ciliate ancestry does not explain the general occurrence of
flagellated sperm in metazoans. No comparable cells are produced in ciliates, and
it is necessary to assume a de novo origin of motile sperm in the metazoan
ancestor. The most serious objection to the Syncytial Theory is the necessity for
making the acoels the most primitive living metazoans. Bilateral symmetry then
becomes the primitive symmetry for metazoans, and the radially symmetrical
cnidarians must be derived secondarily from the flatworms. Many specialists now
doubt that acoels are even the most primitive flatworms (p. 185).
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 72 of 10
The Colonial Theory, in which the metazoans are derived by way of a
colonial flagellate, is the classic and most frequently encountered theory of the
origin of multicellular animals. There is increasing evidence in its support, and it is
the most widely held view among contemporary zoologists. This idea was first
conceived by Haeckel (1874), later modified by Metschnikoff (1887), and revived
by Hyman (1940). The Colonial Theory maintains that the flagellates are the
ancestors of the metazoans, and in support of such an ancestry the following facts
are cited as evidence. Flagellated sperm cells occur throughout the Metazoa.
Flagellated body cells commonly occur among lower metazoans, particularly
among sponges and cnidarians. True sperm and eggs have evolved in the phytoflagellates. The phytoflagellates display a tendency toward a type of colonial
organization that could have led to a multicellular construction; in fact, a
differentiation between somatic and reproductive cells has been attained in Volvox.
Although VoiVox is frequently used as a model for the design of the flagellate
colonial ancestor, these autotrophic organisms with plantlike cells are not likely
ancestors of metazoans. Ultrastruc-tural evidence points to the choanoflagellates, a
small group of animal-like, monoflagellated pro-toza, as the best candidates. Some
are solitary and some are colonial.
The Colonial Theory holds that the ancestral metazoan probably arose from
a spherical, hollow, colonial flagellate. Like Volvox, the cells were flagellated on
the outer surface; the colony possessed a distinct anterior-posterior axis and swam
with the anterior pole forward; and there was a differentiation of somatic and
reproductive cells. This stage was called the blastaea in Haeckel's original theory,
and the hollow blastula, or coeloblastula, was considered a recapitulation of this
stage in the embry-ogeny of living metazoans (Fig. 3-9). According to Haeckel, the
blastaea invaginated to produce, a double-walled, saclike organism, the gastraea
(Fig. 3-9). This gastraea was the hypothetical metazoan ancestor, equivalent to the
gastrula stage in the embryonic development of living metazoans.
Blastaea
Blastaea
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 73 of 10
Figure 3-9 Hypothetical stages in the evolution of early metazoans according to
Haeckel (left), Metschnikoff (middle), and Grell-Butschli (right). (Greatly modified fromGrell, 1981.)
In addition to embryological evidence, Haeckel noted the close structural similarity
between the gastraea and some lower metazoans, such as the hydrozoan cnidarians
and certain sponges. Both of these latter organisms are double walled, with a single
opening into a saclike cavity.
Haeckel's blastaea and gastraea stages are still widely held as starting points
in metazoan evolution and have been recently elaborated in the phylogenetic
scheme of Nielsen (1985).
A popular revision of Haeckel's theory still encountered today was initiated
by Metschnikoff (1887), who noted that the primitive mode of gas-trulation in
cnidarians is by ingression, in which cells are proliferated from the blastula wall
into the interior blastocoel. This produces a solid gastrula. Invagination may have
been a secondary embryonic shortcut. Metschnikoff therefore argued that through
the migration of cells into the interior, the originally hollow sphere (blastaea)
became transformed into an organism having a solid structure (gastraea) (Fig. 3-9).
The body of this hypothetical ancestral metazoan is believed to have been ovoid
and radially symmetrical. The exterior cells were flagellated and, as such, assumed
a locomotor sensory function. The solid mass of interior cells functioned in
nutrition and reproduction. There was no mouth, and food could be engulfed
anywhere on the exterior surface and passed to the interior. Since this hypothetical
organism is very similar to the planula larva of cnidarians, it has been called the
planuloid ancestor.
From such a free-swimming, radially symmetrical, planuloid ancestor the
lower metazoans are believed to have arisen. On the basis of this theory, the
primary radial symmetry of the cnidarians can thus be accounted for as being
derived directly from the planuloid ancestor. The bilateral symmetry of the
flatworms would then represent a later modification in symmetry.
2. Class calcareous sponge-Calcarea
Members of this class, known as calcareous sponges, are distinctive in having
spicules con posed of calcium carbonate. All the spicules are of the same general
size and are monaxons or three or four-pronged types; they are usually separate.|
Spongin fibers are absent. All three grades of structure—asconoid, syconoid, and
leuconoid are encountered. Many Calcarea are drab, although briliant yellow, red,
and lavender species are known] They are not as large as species of other class
most are less than 10 cm in height. Species of сalcareous sponges exist throughout
the oceans of the world, but most are restricted to relatively shallow | coastal
waters. Genera such as Leucosolenia and Sycon are commonly studied examples
of asconoidi and syconoid sponges.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 74 of 10
The subclass Sphinctozoa contains a single recently discovered
representative (Neocoelia) from | shaded recesses on Indo-Pacific reefs. The
Sphinetozoa were abundant from the late Paleozi through the Mesozoic. There are
no spicules, but a calcareous skeleton forms an outer perforated wall and also the
walls of interior chambers.
2. Class Glass sponges - Hyalospongia
Class Hexactinellida, or Hyalospongiae
Representatives of this class are commonly knows as glass sponges. The
name Hexactinellida is rived from the fact that the spicules include a hexaxon, or
six-pointed type (Fig. 4-5G). Furthermore, some of the spicules often are fused to
form a skeleton that may be lattice-like and built of long, siliceous fibers. Thus,
they are called glass spong The glass sponges, as a whole, are the most syi metrical
and the most individualized of sponges—that is, they show less tendency to ft
interconnecting clusters or large masses with oscula. The shape is usually cup-,
vase-, orun and they average 10 to 30 cm in height. The oring in most of these
sponges is pale. There is well-developed atrium, and the single osculum is
sometimes covered by a sieve plate—a gratelikej covering formed from fused
spicules. Lattice-like skeletons composed of fused spicules in sperieJ such as
Venus's-flower-basket (Euplectella) n the general body structure and symmetry of
thehvl ing sponge and are very beautiful; the white, filmy] skeleton looks as if it
were fashioned from wool . Basal tufts of spicule fibers implanted in sand or
sediments adapt many species for living on soft bottoms.
The histology of hexactinellids is very different from that of other sponges.
All surfaces exposed to water are covered not by pinacoderm but by a syncytial
layer (trabecular syncytium), through which long spicules may project. Another
syncytium, containing flagella with collars, lines the flagellated chambers.
Archeocytes are one of the few discrete cell types. The flagellated chambers are
commonly thimble shaped and oriented at right angles in parallel planes to the
body wall and central antrium. Hexactinellids are thus somewhat syconoid in
structure.
In contrast to the Calcarea, the Hexactinellida are chiefly deepwater sponges.
Most live between depths of 200 and 1000 meters, but some have been dredged
from the abyssal zone. Although found throughout the world, hexactinellids are the
dominant sponges in the Antarctic.
Species of Euplectella, Venus's-flower-basket, display an interesting
commensal relation with certain species of shrimp (Spongicola). A young male and
a young female shrimp enter the atrium and, after growth, are unable to escape
through the sieve plate covering the osculum. Their entire life is spent in the
sponge prison, where they feed on plankton brought in by the sponge's water currents. A spider crab (Chorilla) and an isopod (Aega) are also found as commensals
with some species of Euplectella.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 75 of 10
3. Class Demospongiae
This large class contains 90 per cent of sponge species and includes most of
the common and familiar forms. These sponges range in distribution from shallow
water to great depths.
Coloration is frequently brilliant because of pigment granules located in the
amebocytes. Different species are characterized by different colors, and a complete
array of hues is encountered.
The skeleton of this class is variable. It may consist of siliceous spicules or
spongin fibers or a combination of both. The genus Oscarella is unique in lacking
both a spongin and a spicule skeleton. These Demospongiae with siliceous skeleton differ from the Hexactinellida in that their larger spicules are monoaxons or
tetraxons, never hexaxons. When both spongin fibers and spicules are present, the
spicules are usually connected to, or completely embedded in, the spongin fibers.
All Demospongiae are leuconoid, and the majority are irregular, but all types
of growth patterns are displayed. Some are encrusting (Fig. 4-8E);
some have an upright branching habit or form irregular mounds; others are
stringlike or foliaceous (Fig. 4-8C). There are also species, such as Poteiion (Fig.
4-8D), that are goblet or urn shaped, and others, such as Callyspongia (Fig. 4-8B),
that are tubular. The great variation in the shapes of the Demospongiae reflects, in
part, adaptations to limitations of space, inclination of substrate, and current
velocity. Large upright forms can exploit vertical space and use only a small part
of their surface area for attachment. Encrusting forms, although they require more
surface area for attachment, can utilize vertical surfaces and very confined habitats,
such as crevices and spaces beneath stones (Fig. 4-2). The largest sponges are
members of the Demospongiae; some of the tropical loggerhead sponges
(Spheciospongia) form masses over a meter in height and diameter.
Several families of Demospongiae deserve mention. The boring sponges,
composing the family Clionidae, are able to bore into calcareous structures, such as
coral and mollusk shells (Fig. 4-2), forming channels that the body of the sponge
then fills. At the surface the sponge body projects from the channel opening as
small papillae. These papillae represent either clusters of ostia opening into an
incurrent canal or an osculum. Excavation, which is begun by the larva, occurs
when special amebocytes remove chips of calcium carbonate. The amebocyte
begins the process, etching the margins of the chip by digesting the organic framework material and dissolving the calcium carbonate (Pomponi, 1979) (Fig. 4-14B).
The chip is then undercut in the same manner, the amebocyte enveloping the chip
in the process. Eventually, the chip is freed and is eliminated through the excurrent
water canals. Cliona celata, a common boring sponge that lives in shallow water
along the Atlantic coast, inhabits old mollusk shells. The bright sulfur yellow of
the sponge is visible where the bored channels reach the surface of the shell.
Cliona lampa of the Caribbean is red, and it commonly overgrows the surface of
the coral or coralline rock that it has penetrated as a thin encrusting sheet. Boring
sponges are important agents in the decomposition of shell and coral (Fig. 4-14).
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 76 of 10
Members of two families of sponges occur in fresh water, but the family
Spongillidae contains the majority of freshwater species. The Spongillidae are
worldwide in distribution and live in lakes, streams, and ponds where the water is
not turbid. They have an encrusting growth pattern, and some are green because of
the presence of symbiotic zoochlorellae in the amebocytes. The algae are brought
in by water currents and are transferred from the choanocytes to the amebocytes.
The growth rate of sponges deprived of zoochlorellae is less than half the normal
rate (Frost and Williamson, 1980).
Many marine sponges, both Demospongiae and Calcarea, are now known to
harbor symbiotic organisms. A few species contain nonmotile dinoflagellates
(zooxanthellae), but the most common symbionts are cyanobacteria (blue-green
algae), which live within the mesohyl or within specialized amebocytes. The
cyanobacterial symbionts of some keratose sponges, including Verongia, may
make up more than 33 per cent of the sponge. Such sponges live in shallow, welllighted habitats. Excess photosynthate in the form of glycerol and a phosphorylated
compound are utilized by the sponge host. Although bacteria filtered from the
water currents are an important part of the sponge diet, there is no evidence that the
symbiotic bacteria are digested (Vacelet, 1979; Wilkinson, 1978, 1979, and 1983).
The family Spongiidae contains the common bath sponges. The skeleton is
composed only of spongin fibers. Spongia and Hippospongia, the two genera of
commercial value, are gathered from important sponge-fishing grounds in the Gulf
of Mexico, the Caribbean, and the Mediterranean. (There is no longer any large,
commercial sponge fishing in the United States.) The sponges are gathered by
divers, and the living tissue is allowed to decompose in water. The remaining
undecomposed sket-j eton of anastomosing spongin fibers is then washed I (Fig. 4
4K). The colored block "sponges" seen of store counters are a synthetic product.
Class Sclerospongiae
A fourth class of sponges, the Sclerospongiae, con-l tains a small number of
species found in grottoj and tunnels associated with coral reefs in various I parts of
the world (Jackson et al, 1971). These leu- Г conoid sponges differ from other
sponges in having I an internal skeleton of siliceous spicules andspoM gin fibers
and an outer encasement of calcium caiT bonate. Гһе numerous oscula are raised
on Л calcareous skeletal mass and have a starlike configuration from the
converging excurrent canals | (Hartman and Goreau, 1970).
Summary
1 Sponges are sessile aquatic animals, mostly marine and largely inhabitants of
hard substrates.
2 They are primitive in their lack of organs, including mouth and gut. There are
different kinds of cells, but cellular differentiation has not followed the common
designs of other animals.
76 The Sponges
3 The bodies of sponges are organized around a system of water canals, a
specialization correlated with sessility.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 77 of 10
4 The small, vase-shaped asconoid body form, in which flagellated choanocytes
line an interior atrial chamber, is the primitive sponge form. The evolution of the
common leuconoid form, in which the flagellated cells are distributed within a vast
number of minute chambers, has permitted the attainment of much larger size and
great diversity of shape, since each addition to the sponge body brings with it all of
the units necessary to provide the required additional water flow.
5 The growth form of sponges is, in part, an adaptive response to the availability of
space, the inclination of the substrate, and the current velocity.
6 Support is provided by a skeleton of organic spongin fibers or siliceous or
calcareous spicules, or a combination of spongin fibers and siliceous spicules.
7 Feeding, gas exchange, and waste removalde pend on the flow of water through
the body. Thi ability of the choanocyte collar to remove d tremely small particles
from the water stream has probably been an important factor in the long, successful
history of sponges.
8 Probably because of their sessility, mosi sponges are hermaphroditic. Sperm
leave Offi sponge and enter another in the currents flowing through the water
canals. Eggs in the mesophylan fertilized in situ. They may then be released by wq
of the water canals or brooded up to the larva stage. In most sponges the flagellated
larva is a blastula, and reorganization equivalent to gastrulatioin occurs following
settling.
9 Sponges are probably an early evolution! side branch that gave rise to no other
groups of animals.
Lecture #5. Species diversity and structural features of the type Coelenterates.
1. Increase the general level of organization cnidarians compared with sponges.
2. General characteristics of the type of coelenterates. Class Hydroids.
3. Class Scyphozoa-Scyphozoa. Coral polyps class-Antozoa.
1. Increase the general level of organization cnidarians compared with
sponges.
The Cnidarians and Ctenophores
The phylum Cnidana, or Coelenterata, includes the familiar hydras, jellyfish,
sea anemones, and corals. The bright coloring of many species, combined with a
radial symmetry, creates a beauty that is surpassed by few other animals. The
radial symmetry is commonly considered justification for uniting the cnidarians
and the ctenophores within a division of phyla of the Animal Kingdom called the
Radiata.
The cnidarians possess two basic metazoan structural features. There is an
internal space for digestion, called in cnidarians a gastrovascular cavity (Fig. 5-1).
This cavity lies along the polar axis of the animal and opens to the outside at one
end to form a mouth. The presence of a mouth and digestive cavity permits the use
of a much greater range of food sizes than is possible in the protozoa and sponges.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 78 of 10
In cnidarians a circle of tentacles, representing extensions of the body wall,
surrounds the mouth to aid in the capture and ingestion of food.
The cnidarian body wall consists of three basic layers (Fig. 5-1): an outer
layer of epidermis, an inner layer of cells lining the gastrovascular cavity, and
between these a layer called mesoglea. Thei soglea ranges from a thin, noncellular
membranetl a thick, fibrous, jelly-like, mucoid material with Л without wandering
cells. The mesoglea probably evolved from a basement membrane, and in forms
like Hydra it has hardly progressed beyond thJ level. In others it is more like
connective tissue] but the cells appear to be derived from the epidel mis. Thus,
cnidarians are diploblastic; i.e., tbl body is constructed from only two germ layers,
eel toderm and endoderm.
Histologically, the cnidarians have remained I rather primitive, although
they anticipate some el the specializations that are found in higher metazoans. A
considerable number of cell types conJ pose the epidermis and gastrodermis, but
there J only a limited degree of organ development.
Although all cnidarians are basically tentaculate and radially symmetrical, two
structural types are encountered within the phylum. One type, which is sessile, is
known as a polyp. The other form is free swimming and is called a medusa.
Typically, the body of a polyp is a tube or cylinder, in which the oral end, bearing
the mouth and tentacles, is directed upward, and the opposite, or aboral, end is
attached (Fig. 5-L4).
The medusoid body resembles a bell or an umbrella, with the convex side
upward and the mouth located in the center of the concave undersurface (Fig. 5IB).
The tentacles hang down from the margin of the bell. In contrast to the polypoid
mesoglea (middle layer), which is more or less thin, the medusoid mesoglea is
extremely thick and constitutes the bulk of the animal. Because of this mass of
jelly-like mesogleal material, these cnidarian forms arc commonly known as
jellyfish. Some cnidarians exhibit only the polypoid form, some only the medusoid
form, and others pass through both in their life cycle. Colonial organization has
evolved numerous times within the phylum, especially in polypoid forms.
Except for the hydras and a few other freshwater hydrozoans, cnidarians are
marine. Most are inhabitants of shallow water; sessile forms abound on rocky
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 79 of 10
coasts or on coral formations in tropical waters. The phylum is composed of
approximately 9000 living species, and a rich fossil record dates from the
Cambrian period.
2. General characteristics of the type of coelenterates. Class Hydroids.
SUMMARY
1 Cnidarians are aquatic, radially symmetrical animals with tentacles encircling the
mouth at one end of the body. The mouth is the only opening into the gut cavity.
2 Cnidarians exhibit two body forms: the medusa, which is adapted for a pelagic
existence, and the polyp, which is adapted for an attached, benthic existence.
Colonial organization has evolved in many polypoid groups.
3 The body wall consists of an outer epidermis, an inner gastrodermis, and an
intervening mesoglea. The latter may be thin or thick, acellularor cellular.
4 Cnidarians are primitive in their lack of orl gans theii lack oi fully differentiated
epithelil and muscle cells, and the diploblastic origin of Л adult body.
5 Most feed on zooplankton, although son utilize larger animals and some are
suspension! feeders on fine particulate matter. Prey is caugbJ with the tentacles and
immobilized by ехркші cells, called cnidocytes, which are unique to tfj phylum.
Digestion is initially extracellular, thd intracellular.
6 The neurons arc usually arranged as a nervel net at the base of the epidermal and
gastrodenol layers, and impulse transmission tends to be ] dialing. Synaptic
junctions are common^ nonpolarized.
7 A ciliated, free-swimming stereogastrula, I called the planula larva, occurs in the
life cycleif most cnidarians.
The class Hydrozoa contains about 2700 species of common cnidarians, but
because of their small size and plantlike appearance, the layman is largely unaware
of their existence. A considerable part of the marine growth attached to rocks,
shells, and wharf pilings, usually dismissed as "seaweed," is frequently composed
of hydrozoan cnidarians.
The few known freshwater cnidarians belong to to the class Hydrozoa. They
include the hydras and some small, freshwater jellyfish.
Hydrozoans display either the polypoid or the medusoid structure, and some
species pass through both forms in their life cycle. Three characteristic unite the
members of this class. The mesoglea is never cellular; the gastrodermis lacks
cnidocytes and the gonads are epidermal, or if gastrodermal, the eggs and sperm
are shed directly to the outside and not into the gastrovascular cavity.
SYSTEMATIC RESUME OF CLASS HYDROZOA
Order Trachylina. Medusoid hydrozoans lacking a polypoid stage. Medusa
develops directly from an actinula. This order contains perhaps the most primitive
members of the class. Liriope, Aglaura.
Order Hydroida. Hydrozoans with a well-developed polypoid generation.
Medusoid stage present or absent. The majority of hydrozoans belong to this order.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 80 of 10
Suborder Limnomedusae. Mostly freshwater hydrozoans possessing small solitary
polyps and free medusae. The marine Gonionemus; the freshwater Craspedacusta.
Suborder Anthomedusae. Skeletal covering, when present, does not surround
hydranth (athecate). Free medusae, which are tall and bell-shaped, are commonly
present. Tubularia, Vermana, Syncoryne, Eudendrium, Hydracti-nia, Polyorchis,
Branchiocerianthus, the freshwater hydras.
Suborder Leptomedusae. Hydranth surrounded by a skeleton (thecate). Free
medusae are commonly absent, but when present, they are more or less flattened.
Obelia, Campanularia, Abie-tinaria, Sertularia, Plumularia, Aglaophenia. Suborder
Chondrophora. Pelagic, polymorphic, polypoid colonies. (These cnidarians can
also be interpreted as large, single, inverted polyps.) Velella, Porpita. Order
Actinulida. Tiny, solitary hydrozoans resembling actinula larvae. No medusoid
stage present. Interstitial inhabitants. Halammohydra, Oto-hydra.
Order Siphonophora. Pelagic hydrozoan colonies of polypoid and medusoid
individuals. Colonies with float or large swimming bells. Largely in warm seas.
Physalia (Portuguese man-of-war), Stephalia, Nectalia. Order Hydrocorallina.
Colonial polypoid hydrozoans that secrete a calcium carbonate skeleton. Suborder
Milleporina. Stinging, or fire, coral. Skeleton covered by only a thin epidermal
layer. Defensive polyps arising from separate pores encircling a central
gastrozooid. MiJIe-pora is the only genus.
Suborder Stylasterina. A thick layer of tissues overlying skeleton. Defensive and
feeding polyps located within star-shaped openings on the skeleton. Stylaster,
Allopora.
SUMMARY
1 Members of the class Hydrozoa are medusoid or polypoid or exhibit both forms
in their life cycle. The mesoglea is acellular, cnidocytes are restricted to the
epidermis, and gametes develop in the epidermis. Hydrozoans may be the most
primitive of the three classes of cnidarians.
2 Hydromedusae are usually small and planktonic.
3 The most primitive hydrozoans are probably medusoid species, in which the
pelagic actinula develops directly into an adult medusa. Such a life cycle may also
be primitive for the phylum.
4 The polypoid form may have arisen in some medusoid species in which the
actinula passed through a period of attachment prior to development into a pelagic
adult; i.e., the attached actinula was the first polyp.
5 Early polypoid stages, including the attached actinula, probably reproduced
asexually by budding. Persistent attachment of the buds led to colonial polypoid
species, called hydroids, which now compose the majority of hydrozoans.
6 Associated with colonial organization has been the evolution of a skeleton
(support) and polymorphism (division of labor).
7 Naked solitary species, such as hydras and the Gonionemus polyp, probably stem
from early polypoid forms that were not colonial.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 81 of 10
8 Suppression of the medusa through attachment to the polyp and subsequent
reduction has evolved independently in different hydrozoan lines, and living
species exhibit all degrees of reduction in the medusoid form.
3. Class Scyphozoa-Scyphozoa. Coral polyps class-Antozoa.
Scyphozoans are the cnidarians most frequently referred to as jellyfish. In
this class the medusa is the dominant and conspicuous individual in the life cycle;
the polypoid form is restricted to a small larval stage. In addition, scyphozoan
medusae are generally larger than hydromedusae. The majority of scyphozoan
medusae have a bell diameter ranging from 2 to 40 cm; some species are even
larger.
SYSTEMATIC RESUME OF CLASS SCYPHOZOA
Order Stauromedusae, or Lucernariida. Sessile polypoid scyphozoans attached
by a stalk on the aboral side of the trumpet-shaped body. Chiefly in cold littoral
waters. Haliclystus, Craterolophus, Lucernaria.
Order Coronatae. Bell of medusa with a deep encircling groove or constriction,
the coronal groove, extending around the ex-umbrella. Many deep-sea species.
Periphylla, Stephanoscyphus, Nausithoe, Linuche, Atolla.
Order Semaeostomeae. Scyphomedusae with bowl-shaped or saucer-shaped bells
having scalloped margins. Manubrium divided into four oral arms. Gastrovascular
cavity with radial canals or channels extending from central stomach to bell margin. Occur throughout the oceans of the world, especially along coasts. Cyanea,
Pelagia, Amelia, Chrysaora, Stygiomedusa.
Order Rhizostomeae. Bell of medusa lacking tentacles. Oral arms of manubrium
branched and bearing deep folds into which food is passed. Folds, or "secondary
mouths," lead into arm canals of manubrium, which pass into stomach. Original
mouth lost through fusion of oral arms, except in Stomolophus. Mostly tropical
and subtropical shallow water scyphozoans. Cassiopea, Rhizostoma, Mastigias,
Stomolophus.
Although the Cubomedusae have, in this edition, been discussed with the
Scyphozoa, the nature of their nematocysts, the possession of a velum, and their
life cycle are considered evidence that they are not closely related to the other scyphozoans and should be placed within a separate class, the Cubozoa (Werner,
1975; Calder and Peters, 1975).
Class Cubomedusae. Medusoid cnidarians with bells having four flattened sides.
Bell margin simple and bearing four tentacles or tentacle clusters. An attached
polypoid larva follows planula. Tropical and subtropical oceans. Carybdea, Chiropsalmus, Chironex.
SUMMARY
1 Members of the class Scyphozoa are pelagic cnidarians in which the medusa is
the dominant and conspicuous form. A polypoid larva, equivalent to an actinula,
follows the planula. Assuming the primitive nature of the medusoid form, scy-
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 82 of 10
phozoans are primitive in their life cycle and perhaps evolved early from the
ancestral hydrozoans.
2 Within the Scyphozoa, specialization has led to complexities in medusoid
structure, as evidenced by such features as the following: larger size than that of
most hydromedusae, more highly developed manubrium, cellular mesoglea,
septate gut or at least a gut with gastric filaments, gastrodermal cnidocytes, and
some development of sense organs.
3 The gonads are gastrodermal, and the eggs, which are shed through the mouth,
develop into planula larvae. Following settling, the planulae develop into polypoid
larvae, which feed and may reproduce asexually.
4 In some species the polypoid larva transforms directly into a young medusa,
which can be taken as additional evidence that the polypoid form was derived from
a larval stage in the evolution of the cnidarians. In most species of scyphozoans,
young medusae are budded off transversely from the oral end of the polypoid
larva.
Class Anthozoa
This is the largest of the сnidarian classes and contains over 6000 species,
Although the anthozoans are polypoid, they differ considerably from hydrozoan
polyps The I mouth leads into a tubular pharynx that cxtendil more than halfway
into the gastrovascular cavil (Fig. 5-34). The gastrovascular cavity is divideded
longitudinal mesenteries, or septa, into radiating compartments, and the edges of
the mesentery bear nematocysts. The gonads, as in the scyphozoans are
gastrodermal, and the fibrous mesoglea contains cells. The nematocysts, unlike
those of hydrozoans and scyphozoans, do not possess an opercullum, or lid. Some
anthozoan nematocysts have a | three-part tip that folds back on expulsion; іn
оthers, the thread appears to rupture directly through) the end of the capsule.
In order to simplify the survey of this class, we will deal with the sea anemones,
the stony corals and the octocorallian corals separately.
SUMMARY
1 Members of the class Anthozoa are polypoid cnidarians; the medusoid stage is
entirely lacking.
2 The anthozoan polyp is more specialized than that of hydrozoans, and its cellular
mesoglea, septate gastrovascular cavity, cnidocytes in gastric filaments, and
gastrodermal gonads indicate a closer phylogenetic relationship with the Scyphozoa than with the Hydrozoa.
3 The difference in the body form of the Scyphozoa and the Anthozoa (medusa
versus polyp) may be reconciled if the anthozoans are derived through the
polypoid larva of scyphozoans.
4 The two subclasses, the Zoantharia and the Octocorallia, reflect different levels
of structural evolution within the Anthozoa. The Octocorallia have retained an
arrangement of eight complete mesenteries and eight tentacles, which may be the
primitive anthozoan condition. Colonial organization is characteristic of almost all
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 83 of 10
octocorallians, and the polyps are interconnected through a complex mass of
mesoglea and gastrodermal tubes. The zoantharia display a more complex system
of mesenteries, which always exceed eight in number. There are many solitary
forms, and colonial species are connected by more or less simple outfoldings of the
body wall.
5 Sea anemones are the principal group of solitary anthozoans, and perhaps
because of their solitary condition, many species have evolved a larger size than
most other anthozoan polyps. The number and complexity of their mesenteries,
providing a large surface area of gastric filaments, may be related to the utilization
of larger prey.
6 The majority of .anthozoans are colonial, and this type of organization has
evolved independently a number of times within the class. Although colonies may
reach a large size, the individual polyps are generally small. There are some groups
with polymorphic colonies, but this condition is not as widespread as in the
hydrozoans.
7 Scleractinian corals, although similar to sea anemones, are largely colonial and
are unique in their secretion of an external calcareous skeleton. The skeleton
provides the colony with a uniform substrate on which the living colony rests. The
sclerosepta may contribute to the adherence of the polyps within the thecal cups
and provide some protection against grazing predators when the polyps are
withdrawn.
8 The majority of scleractinian corals are tropical reef inhabitants (hermatypic) and
harbor zooxanthellae. Zooxanthellae are found in many other anthozoans as well as
some scyphomedusae and some hydrozoans.
9 The colonial alcyonaceans, or soft corals, which are most abundant on IndoPacific reefs, in many ways parallel the scleractinian corals, for the massive
coenenchymal mass forms the substrate from which the individual polyps arise.
10 The branching, rodlike colonies or gorgonian corals are adapted for exploiting
the vertical water column while using only a small area of the substrate for
attachment. Flexible support is provided by a central, organic skeletal rod and
separate calcareous spicules embedded in the coenenchyme.
11 The pennatulaceans, which include sea pens, sea feathers, and sea pansies, are
adapted for life on soft bottoms. A large, primary polyp, which determines the
form of the colony, not only provides anchorage in the sand but also acts as the
substrate from which the small, secondary polyps arise.
12 A planula larva is characteristic of most anthozoans and develops into the
polyp. Colonial forms are derived by budding from the first polyp.
Lecture # 6. Section right - symmetric animals. Several protostomes.
Systematic position worms, external morphology, covers.
1. Systematic position type worm flat, round and Annelida.
2. The external morphology of of flat, round and annelids.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 84 of 10
3. Distinctive features of flat sheets, and round worms.
1.Systematic position type worm flat, round and Annelida
Classification
Kingdom:
Sub- Kingdom:
Phylum:
Phylum:
Phylum:
Animalia
Metazoa
Plathyhelminthes
Nemathelminthes
Annelida
What are flatworms?
Flatworms are soft, flattened worms that have tissues and internal organs. They
are the simplest animals to have:
three germ layers – in other words they are triploblastic,
bilateral symmetry, and
cephalization.
Flatworms are also known as acoelomates, meaning without a coelom. A
coelom is a fluid filled body cavity that is lined with tissue derived from the
mesoderm. No coelom forms between the tissues of flatworms.
Groups of Flatworms
The three main groups of flatworms include the:
Turbellaria (freeliving),
Cestoda (parasitic tapeworms), and
Trematoda (parasitic flukes).
Phylum NEMATHELMINTHES (=NEMATODA)
The phylum was created by Gegenbaur in 1859 for unsegmented round worms
but now phylum Nematoda is commonly used instead. The phylum includes
bilaterally symmetrical triploblastic and pseudocoelomate animals with organ
system of organization. Excretion involves a giant excretory cell called Renette
cell. They have tube-within-tube type of body plan.
Class NEMATODA
It includes round worms that are both free living and parasitic, containing
about 28,000 species, of which 16,000 are parasitic. They are slender and
unsegmented worms having bilateral symmetry and their skin consists of a
syncytium covered by a thick layer of cuticle. They possess only longitudinal
muscles. Most of the species are dioecious and lay shelled eggs.
Phylum Annelida
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 85 of 10
The Annelida are a medium sized phylum of more than 9,000 species of
worms. Most species prefer aquatic environments, but there are also a number of
well know terrestrial species. Only a few species of annelids are commonly known
to human beings, these include the delightful Rain, Dew or Earthworms that work
so hard to make our soils healthy, the Ragworms and Lugworms used by marine
fishermen and the much smaller Tubifex or Red worms used by aquarists to feed
their fish. In many countries people are still familiar with Medicinal leeches, and
people who live closer to nature are naturally more familiar with a much wider
range of Annelids than those who live in cities.
The Phylum Annelida is divided into 3 classes, one of which the Clitellata
could really be called a Superclass, it contains three subclasses, the Oligochaeta,
the Branchiobdella and the Hirundinea. The other two classes are the Polychaeta
which contains the largest number of species and the Aelosomatida which contains
very few.
2. The external morphology of flat, round and annelids
Flatworm, also called platyhelminth, any of the phylum Platyhelminthes, a
group of soft-bodied, usually much flattened invertebrates. A number of flatworm
species are free-living, but about 80 percent of all flatworms are parasitic—
i.e., living on or in another organism and securing nourishment from it. They are
bilaterally symmetrical (i.e., the right and left sides are similar) and lack
specialized respiratory, skeletal, and circulatory systems; no body cavity (coelom)
is present. The body is not segmented; spongy connective tissue (mesenchyme)
constitutes the so-called parenchyma and fills the space between organs.
Flatworms are generally hermaphroditic—functional reproductive organs of both
sexes occurring in one individual. Like other advanced multicellular animals, they
possess three embryonic layers—endoderm, mesoderm, and ectoderm—and have a
head region that contains concentrated sense organs and nervous tissue (brain).
Most evidence, however, indicates that flatworms are very primitive compared
with other invertebrates (such as the arthropods and annelids). Some modern
evidence suggests that at least some flatworm species may be secondarily
simplified from more complex ancestors.
Nematodes are the most speciose phylum after the arthropods, they occur in nearly
every habitat including as parasites in all sorts of plants and animals, (they don't
like dry places however).
Characteristics of Nematoda:- Bilaterally symmetrical, and vermiform; body
has more than two cell layers, tissues and organs; body cavity is a pseudocoel,
body fluid under high pressure; body possesses a through gut with a subterminal
anus; body covered in a complex cuticle; has a nervous system with pharyngeal
nerve ring; has no circulatory system (no blood system); reproduction normally
sexual and gonochoristic; feed on just about everything; live just about
everywhere, many species are endoparasites.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 86 of 10
Annelida [Lat., anellus = a ring], phylum of soft-bodied, bilaterally symmetrical
(see symmetry, biological), segmented animals, known as the segmented, or
annelid, worms. Over 12,000 known species are grouped in three classes:
the earthworms and freshwater worms (oligochaetes), the leeches (hirudineans),
and the marine worms (polychaetes). Annelids are found throughout the world,
from deep ocean bottoms to high mountain glaciers. They live in protected habitats
such as mud, sand, and rock crevices, and in and among other invertebrate animals,
such as sponges. Many live in tubes they secrete around themselves.
The fundamental characteristic of the phylum is the division of the body into a
linear series of cylindrical segments, or metameres. Each metamere consists of a
section of the body wall and a compartment of the body cavity with its internal
organs. The external divisions, which may be seen in the common earthworm,
correspond to the internal divisions. The annelid body consists of a head region; a
trunk, made up of metameres; and an unsegmented terminal region called the
pygidium. In some primitive members of the phylum the metameres are identical,
or very similar to one another, each containing the same structures; in more
advanced forms there is a tendency toward a consolidation of some segments and a
restriction of certain organs to particular segments. Because of the soft nature of
the annelid body, fossils are not common. Fossils of tube-dwelling polychaetes
have been found, but there is scarcely any fossil record for earthworms and none
for leeches.
3. Distinctive features of flat sheets, and round worms
Flatworms.
The body of flukes is oval to elongate, usually not more than a few cm long,
and the mouth is typically at the anterior end. Adhesive suckers are usually present
around the mouth and may also be present midventrally. The monogenetic flukes
possess large posterior attachment organs, called opisthaptors, provided with
various structures, such as suckers and hooks (Fig. 7-25).
In contrast to the epidermis of the turbellarian, the body of a trematode is covered a
nonciliated cytoplasmic syncytium, the tegument, overlying consecutive layers of
circular, longitudinal, and diagonal muscle. The syncytium represents extensions
of cells that are located in the parenchyma (Fig. 7-26).
The mouth leads into a muscular pharynx that pumps into the digestive tract
the cells and cell fragments, mucus, tissue fluids, or blood of the host on which the
parasite feeds. The pharynx passes into a short esophagus and one or, more
commonly, two blind intestinal ceca that extend posteriorly along the length of the
body (Fig. 7-27A). The physiology of nutrition is still incompletely understood,
but secretive and absorptive cells have been reported, so digestion is apparently
extracellular in part.
The tegument plays a vital role in the physiology of flukes. It provides
protection, especially against the host's enzymes in gut-inhabiting species.
Nitrogenous wastes are passed to the exterior through the tegument, and it is the
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 87 of 10
site of gas exchange. In endoparasites the tegument absorbs some amino acids. The
protein synthesis involved in fluke egg production and in larval reproduction
places especially heavy demands on the amino acid supply.
Nematoda.
The nematode cuticle is considerably more complex than that of other
aschelminths. It contains collagen, as well as other compounds, and it is organized
within three main layers (Fig. 9-14). The outer cortical layer is bounded externally
by a thin epicuticle, which may exhibit quinone tanning. It is typically annulated
(ringed). The median layer varies from a uniform granular structure in some
species to the occurrence of struts, skeletal rods, fibrils, or canals in others. The
basal layer may be striated or laminated or contain spiral fibers.
Growth in nematodes is accompanied by four molts of the cuticle. Beginning at the
anterior end, the old cuticle separates from the underlying epidermis and a new
cuticle is secreted, at least in part. The old cuticle is shed in fragments or intact.
Molting does not occur after the worm becomes adult, but the cuticle continues to
grow.
The epidermis, also called hypodermis, is usually cellular but may be
syncytial in some species. A striking feature of the nematode epidermis is the
expansion of the cytoplasm into the pseudocoel along the middorsal, midventral,
and midlateral lines of the body (Fig. 9-15). The bulging epidermis thus forms
longitudinal cords that extend the length of the body. The epidermal nuclei are
commonly restricted to these cords and are typically arranged in rows.
The muscle layer of the body wall is composed entirely of longitudinal, obliquely
striated fibers arranged in bands, each strip occupying the space between two
longitudinal cords. The fibers may be relatively broad and flat, with the contractile
filaments limited to the base of the fiber, or they may be relatively tall and narrow,
with filaments at the base and sides. In both types the base of the cell containing
the contractile fiber is located against the hypodermis, and the side of the cell with
the nucleus is directed toward the pseudocoel (Fig. 9-16). Each nematode muscle
fiber possesses a slender arm that extends from the fiber to either the dorsal or the
ventral longitudinal nerve cord, where innervation occurs (Fig. 9-15). In most animals a nerve process extends from the nerve cord to the main body of the muscle.
The nematode pseudocoel is spacious and filled with fluid. No free cells are
present, but fixed cells, located either against the inner side of the mm layers or
against the wall of the gut and the inte organs, are characteristic of many
nematodes.
Annelida
Body Wall of Polychaeta.
The polychaete epidermis, or integument, is composed of a single layer of
cuboidal or columnar epithelium, which is covered by a thin collagen cuticle (see
Fig. 10-9 for evolution of cuticle). Mucus-secreting gland cells are a common
component of the epithelium.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 88 of 10
Beneath the epithelium lie, in order, a layer of circular muscle fibers, a much
thicker layer of longitudinal muscle fibers, and a thin layer of peritoneum.
Although the muscles of the body wall essentially comprise two sheaths, the
longitudinal fibers typically are broken up into four bundles—two dorsolateral and
two ventrolateral.
Within the spacious coelom, the gut is suspended by septa and mesenteries.
Thus, each coelomic compartment is divided into right and left halves, at least
primitively. However, the septa have partially or completely disappeared in many
polychaetes.
Body Wall and Coelom of Oligochaeta.
The structure and histology of the oligochaete body wall, especially in
terrestrial species, is essentially like that of burrowing polychaetes. A thin cuticle
overlies an epidermal layer, which contains mucus-secreting gland cells. Circular
muscles are well developed, and the septa partitioning the coelom are relatively
complete. Earthworms, which have the best developed septa, may possess sphincters around septal perforations to control the flow of coelomic fluid from one
segment to another.
In most earthworms each coelomic compartment, except at the extremities, is
connected to the outside by a middorsal pore located in the intersegmental furrows
and provided with a sphincter. These pores exude coelomic fluid, which aids in
keeping the integument moist. When disturbed, some giant earthworms squirt fluid
several centimeters.
Body Wall and Coelom of Hirudinea.
The body wall contains a more distinct connective tissue dermis than is
present in other annelids, and some of the unicellular gland cells of the integument
are very large and sunken into the connective tissue layer (Fig. 10-63). The
longitudinal muscle layer of the body wall is powerfully developed, but there are
also circular, oblique, and dorsoventral muscle fibers.
Lectura # 7. System metabolism. The structure of the digestive system of flat,
round and annelids.
1. System of metabolism in flat, round and annelids.
2. The structure of the digestive system of flat, round and annelids.
1. System of metabolism in flat, round and annelids.
Metabolism of Flatworms.
Both free-living and parasitic platyhelminths utilize oxygen when it is
available. Most of the parasitic platyhelminths studied have a predominantly
anaerobic metabolism (i.e., not dependent upon oxygen). This is true even in
species found in habitats—such as the bloodstream—where oxygen is normally
available.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 89 of 10
Parasitic platyhelminths are made up of the usual tissue constituents—
protein, carbohydrates, and lipids—but, compared to other invertebrates, the
proportions differ somewhat; i.e., the carbohydrate content tends to be relatively
high and the protein content relatively low. In larval and adult cestodes,
carbohydrate occurs chiefly as animal starch, or glycogen, which acts as the main
source of energy for species in low oxygen habitats. The level of glycogen, like
other chemical constituents, can fluctuate considerably, depending on the diet or
feeding habits of the host. In some species, more than 40 percent of the worm’s
dried weight is glycogen.
Because carbohydrate metabolism is important in parasitic flatworms, a substantial
amount of carbohydrate must be present in the host diet to assure normal growth of
the parasite. Hence the growth rate of the rat tapeworm (Hymenolepis diminuta) is
a good indicator of the quantity of carbohydrate ingested by the rat. Experiments
have shown that most parasitic worms have the capability of utilizing only certain
types of carbohydrate. All tapeworms that have been studied thus far utilize the
sugar glucose. Many tapeworms can also utilize galactose, but only a few can
utilize maltose or sucrose.
An unusual constituent of both trematodes and cestodes is a round or oval
structure called a calcareous corpuscle; large numbers of them occur in the tissues
of both adults and larvae. Their function has not yet been established, but it is
believed that they may act as reserves for such substances as calcium, magnesium,
and phosphorus.
The chief proteins in cestodes and trematodes are keratin and sclerotin.
Keratin forms the hooks and part of the protective layers of the cestode egg and the
cyst wall of certain immature stages of trematodes. Sclerotin occurs in both cestode
and trematode eggshells, especially in those that have larval stages associated with
aquatic environments.
Platyhelminth eggs hatch in response to a variety of different stimuli in
different hosts. Most trematode eggs require oxygen in order to form the first larval
stages and light in order to hatch. Light is thought to stimulate the release of
an enzyme that attacks a cement holding the lid (operculum) of the egg in place. A
similar mechanism probably operates in cestodes (largely of the order
Pseudophyllidea) whose life cycles involve aquatic intermediate hosts or definitive
hosts, such as birds or fish.
In many cestodes, especially those belonging to the order Cyclophyllidea,
the eggs hatch only when they are ingested by the host. When the host is an insect,
hatching sometimes is apparently purely a mechanical process, the shell being
broken by the insect’s mouthparts. In vertebrate intermediate hosts, destruction of
the shell depends largely on the action of the host’s enzymes. Activation of the
embryo within the shell and its subsequent release depend on other factors,
including the amount ofcarbon dioxide present, in addition to the host’s enzymes.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 90 of 10
Factors involving a vertebrate host are also important in establishing trematode or
cestode infections after encysted or encapsulated larval stages have been ingested.
Under the influence of the same factors, tapeworm larvae are stimulated to
evaginate their heads (i.e., turn them inside out, so to speak), a process that makes
possible their attachment to the gut lining.
Metabolism of Nemathelminthes.
Metabolism of Annelida.
Oligochaeta.
Gas exchange in amost all oligochaetes, aquatic and terrestrial, takes place
by the difi of gases through the general body integi which in the larger species
contains a capillary! work within the outer epidermal layer.
True gills occur in only a few oligochaetes>| cies of the aquatic genera Dero
and Aulophorus have a circle of finger-like gills at the posterior of the body. A
tubificid, Branchiura, has filamentous gills located dorsally and ventrally in the
posterior quarter of the body.
The larger oligochaetes usually have hemoglobin dissolved in the plasma.
The hemoglobin in Lumbricus transports 15 to 20 percent of oxygen utilized
under ordinary burrow condition| where the partial pressure of oxygen is ah same
as that in the atmosphere above ( When the partial pressure drops, the hemoglobin
compensates by increasing its carrying capari| (Weber, 1978).
Many aquatic oligochaetes tolerate relative low oxygen levels and, for a
short period, eve complete lack of oxygen. Members of the far Tubificidae, which
live in stagnant mud and 1 bottoms, are notable examples. There are members of
this family, such as Tubifex tubiex, from long exposure to ordinary oxygen tens
Tubiex ventilates in stagnant water by щ its posterior end out of the mud and
waving about.
Class Hirudinea.
The glossiphoniids and piscicolids (rhynchobdellids) have retained the
blood-vascular system of oligochaetes, but the coelomic sinuses act as a
supplemental circulatory system. In the other leech orders the ancestral circulatory
system has disappeared, and the coelomic sinuses and fluid have been converted to
a blood-vascular system. The hemocoelomic fluid is propelled by the contractions
of the lateral longitudinal channels.
Gills are found only in the Piscicolidae, the general body surface providing
for gas exchange in other leeches. The piscicolid gills are lateral leaflike or
branching outgrowths of the body wall.
Respiratory pigment (extracellular hemoglobin) is found only in the
gnathobdellid and pharyngo-bdellid leeches and is responsible for about half of the
oxygen transport.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 91 of 10
2. The structure of the digestive system of flat, round and annelids.
Type Flatworms.
The mouth leads into a muscular pharynx that pumps into the digestive tract
the cells and cell fragments, mucus, tissue fluids, or blood of the host on which the
parasite feeds. The pharynx passes into a short esophagus and one or, more
commonly, two blind intestinal ceca that extend posteriorly along the length of the
body. The physiology of nutrition is still incompletely understood, but secretive
and absorptive cells have been reported, so digestion is apparently extracellular in
part.
The blind-ending intestine of trematodes consists of a simple sac with an
anterior or midventral mouth or a two-branched gut with an anterior mouth;
an anus is usually lacking, but a few species have one or two anal pores. Between
the mouth and the intestine are often a pharynx and an esophagus receiving
secretions from glands therein. The intestine proper, lined with digestive and
absorptive cells, is surrounded by a thin layer of muscles that effect
peristalsis; i.e., they contract in a wavelike fashion, forcing material down the
length of the intestine. In many larger flukes lateral intestinal branches, or
diverticula, bring food close to all internal tissues. Undigested residue passes back
out of the mouth.
Cestodes have no digestive tract; they absorb nutrients from the host across
the body wall. Most other flatworms, however, have conspicuous digestive
systems.The digestive system of turbellarians typically consists of mouth, pharynx,
and intestine. In the order Acoela, however, only a mouth is present; food passes
directly from the mouth into the parenchyma, to be absorbed by the mesenchymal
cells.
FREE-LIVING FORMS
Free-living platyhelminths (class Turbellaria), mostly carnivorous, are
particularly adapted for the capture of prey. Their encounters with prey appear to
be largely fortuitous, except in some species that release ensnaring mucus threads.
Because they have developed various complex feeding mechanisms, most
turbellarians are able to feed on organisms much larger than themselves, such as
annelids, arthropods, mollusks, and tunicates (e.g., sea squirts). In general, the
feeding mechanism involves thepharynx which, in the most highly developed
forms, is a powerful muscular organ that can be protruded through the mouth.
Flatworms with a simple ciliated pharynx are restricted to feeding on small
organisms such as protozoans and rotifers, but those with a muscular pharynx can
turn it outward, thrust it through the tegument of annelids and crustaceans, and
draw out their internal body organs and fluids. Turbellarians with a more advanced
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 92 of 10
type of pharynx can extend it over the captured prey until the animal is completely
enveloped.
Digestion is both extracellular and intracellular. Digestive enzymes
(biological catalysts), which mix with the food in the gut, reduce the size of the
food particles. This partly digested material is then engulfed (phagocytized) by
cells or absorbed; digestion is then completed within the gut cells.
PARASITIC FORMS
In the parasitic groups with a gut (Trematoda and Monogenea), both
extracellular and intracellular digestion occur. The extent to which these processes
take place depends on the nature of the food. When fragments of the host’s food or
tissues other than fluids or semifluids (e.g., blood and mucus) are taken as nutrients
by the parasite, digestion appears to be largely extracellular. In those that feed on
blood, digestion is largely intracellular, often resulting in the deposition of
hematin, an insoluble pigment formed from the breakdown of hemoglobin. This
pigment is eventually extruded by disintegrating gut cells.
Despite the presence of a gut, trematodes seem able to absorb glucose and
certain other materials through the metabolically active tegument covering the
body surface. Tapeworms, which have no gut, absorb all nutrients through the
tegument. Amino acids (the structural units of proteins) and small molecules
of carbohydrate (e.g., sugars) cross the tegument by a mechanism called active
transport, in which molecules are taken up against a concentration gradient. This
process, similar to that in the vertebrate gut, requires the expenditure of energy.
Cestodes may also be able to digest materials in contact with the tegument by
means of so-called membrane digestion, a little-understood process.
Type Nemathelminthes
Class Nematoda
Many free-living nematodes are carnivorous and feed on small metazoan
animals, including nematodes. Other species are phytophagous, marine and
freshwater species feed on algae, and fungi. Algae and fungi is also important
food sources for many terrestrials species, but there are fungi that trap nematodes.
The worms are caught when they pass through cial hyphal (threadlike) loops,
which close on luxation. A large number of terrestrial nematode pierce the cells of
plant roots and suck out tents. Such nematodes can be responsible for ous damage
to commercial plants. There many deposit-feeding marine, freshwater, and restrial
species, which ingest substratum particles. Deposit feeders and the many
nematodes that live on dead organic matter, such as dung, or on the decomposing
bodies of plants and animals, feed only on associated bacteria and fungi, however.
This is true of the common vinegar eel, Turbatrix aceti, which lives in the sediment
of nonpasteurized vinegar, Nematodes are the largest and most ubiquitous group of
organisms feeding on fungi and bacteria and are of great importance in the food
chains leading from decomposers.
The mouth of the nematode opens into a buccal cavity, or stoma, which is
somewhat tubular and lined with cuticle. The cuticular surface is often
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 93 of 10
strengthened with ridges, rods, or plates, or it may bear a large number of teeth.
The structural details of the buccal cavity are correlated with feeding habits and are
of primary importance in the identification of nematodes. Teeth are especially
typical of carnivorous nematodes; they may be small and numerous or limited to a
few, large, jawlike processes
The feeding habits of Mononchus papillatus, which is a toothed nematode,
have been described by Steiner and Heinly (1922). This terrestrial carnivore, which
has a large dorsal tooth opposed by a buccal ridge, consumes as many as 1000
other nematodes during its life-span of approximately 18 weeks. In feeding, this
nematode attaches its lips to the prey and makes an incision in it with the large
tooth. The contents of the prey are then pumped out by the pharynx.
In some carnivores, as well as in many species that feed on the contents of plant
cells, the buccal capsule carries a long hollow or solid spear (stylet), which can
protrude from the mouth.
Both kinds of stylet are used to puncture prey,« the hollow stylet may act as
a tube through v the contents of the victim are pumped out. In; let-bearing
herbivore, it is used to penetn root cell walls, being thrust rapidly forward i.
backward. Both groups secrete рЛ ryngeal enzymes that initiate digestion of the p
or the plant cell contents and may even aidi penetration of the plant cell wall.
The buccal cavity leads into a tubular pharymJ referred to as the esophagus
by nematologists. The pharyngeal lumen is tnradiateinn section and lined with
cuticle. The* is composed of myoepithelium (as in gastroti and gland cells
(Ruppert, 1982). Frequently,! pharynx contains more than one muscular sweM or
bulb. The pharynx or pharyngeal bulbs act] pumps and bring food from the mouth
intotht| testine. Valves are frequently present.
From the pharynx a long tubular intestine соя posed of a single layer of
epithelial cells cxtcndii| length of the body. A valve located at eache the intestine
prevents food from being forced, of the intestine by the fluid pressure of the f
docoel. A short, cuticle-lined rectum (cloaca in tl male) connects the intestine with
the anus, i is on the midventral line just in front of the j rior tip of the body.
Digestive enzymes are produced by the ph geal glands and the intestinal
epithelium, 1 tion begins extracellularly within the intt lumen but is completed
intracellular (Deua 1978).
Type Annelida
Class Polychaeta.
Nutrition
The feeding methods of polychaetes are closely correlated with the various
life-styles of the class.
Raptorial FEEDERS
Raptorial feeders include members of many family ot surface-dwelling species,
many pelagic groups, tubicolous eunicids and onuphids, and galley dwellers like
the glvcerids and nephtyids. The prey consists of various small invertebrates,
including other polychaetes, which are usually captured by means of an eversible
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 94 of 10
pharynx (proboscis). The pharynx commonly bears two or more horny jaws
composed of tanned protein. The pharynx is rapidly everted; this places the jaws at
the anterior of the body and causes them to open. The food is seized by the jaws
and the pharynx is retracted. Although protractor muscles may be present, an
increase in coelomic pressure resulting from the contraction of body wall muscles
is an important factor in the eversion of the pharynx. When pressure on the
coelomic fluid is reduced, the pharynxHs withdrawn by the retractor muscles,
which extend from the body wall to the pharynx .
Raptorial tube dwellers may leave the tube partially or completely when
feeding, depending on the species. Diopatra uses its hood-shaped tube as a lair.
Chemoreceptors monitor the ventilating current of water passing into the tube, and
when approaching prey is detected, the worm partially emerges from the tube
opening and seizes the victim with a complex pharyngeal armature of teeth. During
feeding the prey may be clasped with the enlarged anterior parapodia. Species of
Diopatra may also feed on dead animals, algae, organic debris, and small
organisms, such as forams, that are in the vicinity of the tube or become attached to
it.
Some raptorial feeders, such as syllids and glycerids, have long, tubular
proboscises. Species of Glycera live within a gallery system constructed in muddy
bottoms. The system contains numerous loops that open to the surface. Lying in
wait at the bottom of a loop, the worm can detect the surface movements of prey
such as small crustaceans and other invertebrates, by changes in water pressure. It
slowly moves to the burrow opening and then seizes the prey with the proboscis.
When the proboscis is retracted, it occupies approximately the first 20 body
segments. At the back of the proboscis are four jaws arranged equi-distantly
around the wall. The proboscis is attached to an S-shaped esophagus. No septa are
present in these anterior segments, and the proboscis apparatus lies free in the
coelom. Just prior to eversion of the proboscis the longitudinal muscles contract
violently, sliding the proboscis forward and straightening out the esophagus. The
proboscis is then everted with explosive force, and the four jaws emerge open at
the tip. Each jaw contains a canal that delivers poison from a gland at the jaw base.
Class Oligochaeta.
Nutrition
The majority of oligochaete species, both aquatic and terrestrial, are
scavengers and feed on dead organic matter, particularly vegetation. Earthworms
feed on decomposing matter at the surface and may pull leaves into the burrow.
They also utilize organic material obtained from mud or soil that is ingested in the
course of burrowing. The food source and feeding habits of earthworms are related
to the species zonation described in the previous section.
Fine detritus, algae, and other microorganisms are important food sources
for many tiny, freshwater species. The common, minute Aeolosoma collects
detritus with its prostomium. The ciliated ventral surface of the prostomium is
placed against the substratum, and the center is elevated by muscular contraction.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 95 of 10
The partial vacuum dislodges particles, which are then swept into the mouth by
cilia. Members of the genus Chaeto-gaster, little oligochaetes that are commensals
on freshwater snails, are raptorial and catch amebas, ciliates, rotifers, and
trematode larvae by a sucking action of the pharynx.
The digestive tract is straight and relatively simple (Fig. 10-52). The mouth,
located beneath the prostomium, opens into a small buccal cavity, which in turn
opens to a more spacious pharynx. The dorsal wall of the pharyngeal chamber is
muscular and glandular and forms a bulb or pad, which is the principal ingestive
organ. In aquatic forms the pharynx is everted and the mucus-covered muscular
disc collects particles on an adhesive pad (Fig. 10-51). In earthworms the pharynx
acts as a pump. Pharyngeal glands produce a salivary secretion containing mucus
and enzymes.
The pharynx opens into a narrow, tubular esophagus, which may be
modified at different levels to form a gizzard or, in lumbricid earthworms,
a crop. In some forms there are two to ten gizzards, each occupying a separate
segment. The gizzard, which is used for grinding food particles, is lined with
cuticle and is very muscular. The crop is thin walled and acts as a storage chamber.
A characteristic feature of the oligochaete gut is the presence of calciferous
glands in certain parts of the esophageal wall. When highly developed, the
glandular region becomes completely separated from the esophageal lumen and
may appear externally as lateral or dorsal swellings (Fig. 10-52). The calciferous
glands are involved in ionic regulation rather than digestion. They function in
ridding the body of excess calcium taken up from food. The calcium is excreted
into the esophagus as calcite, which is not absorbed in transit through the intestine.
The intestine forms the remainder of the digestive tract and extends as a
straight tube through all but the anterior quarter of the body. The anterior half of
the intestine is the principal site of secretion and digestion, and the posterior half is
primarily absorptive. In addition to the usual classes of digestive enzymes, the
intestinal epithelium of earthworms, at least, secretes cellulase and chitinase. The
absorbed food materials are passed to blood sinuses that lie between the mucosal
epithelium and the intestinal muscles. The surface area of the intestine is increased
in many earthworms by a ridge or fold, called a typhlosole, which projects internally from the middorsal walls.
Surrounding the intestine and investing the dorsal vessel of oligochaetes is a
layer of yellowish cells, called chloragogen cells, which play a vital role in
intermediary metabolism, similar to the role of the liver in vertebrates.
Chloragogen tissue is the chief center of glycogen and fat synthesis and storage.
Deamination of proteins, the formation, monia, and the synthesis of urea also take
pk these cells. In terrestrial species silicates obi from food material and the soil are
removed fi the body and deposited in the chloragogen cells waste concretions.
Class Hirudinea.
Nutrition
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 96 of 10
Leeches possess either a proboscis or a sucking pharynx and jaws. The
proboscis (order Rhynchobdellida) is an unattached tube lying within) boscis
cavity, which is connected to the vol mouth by a short, narrow canal proboscis is
highly muscular, has a m lumen, and is lined internally and exten cuticle. Ducts
from large, unicellular glands open into the proboscis. When feet animal extends
the proboscis out of the i forcing it into the tissue of the host.
In jawed leeches (order Gnathobdcllida), lack a proboscis, the mouth is
located in prior sucker. Just with mouth cavity are three large, oval, bladlike each
bearing a large number of small teeth the edge. The three jaws are arranged in a
ma4 one dorsally and two laterally. When the Г feeds, the anterior sucker is
attached to the surf of the prey or host, and the edges of the jaw through the
integument. The Wswing toward and away from each other, ac-mated by muscles
attached to their bases. Salivary gbdssecrete an anticoagulant called hirudin.
Immediately behind the teeth, the buccal cavity opens into a muscular, pumping
pharynx. The er-pobdellids also have a pumping pharynx, but the ware replaced by
muscular folds.
The remainder of the digestive tract is relatively uniform throughout the
class. A short esophagus opens into a relatively long stomach, or crop. The
stomach may be a straight tube, as in the crpobdcl-to,but more commonly it is
provided with 1 to 11 pairs of lateral ceca (Fig. 10-61). Following the stomach is
an intestine, which may be a simple mbeor, as in the rhynchobdellids, may have
tour pairs of slender lateral ceca. The intestine opens into a short rectum, which
empties to the outside though the dorsal anus, located in front of the posterior
sucker.
Many leeches are predacious, but about three fourths of the known species
are bloodsucking ectoparasites. However, in many cases the difference lies only in
the size of the host. The Hirudinidae especially demonstrate a gradation from
predation to parasitism. The Erpobdellidae contain the greatest number of
predacious leeches, but this type of feeding habit is found in other families as well.
Predatory leeches always feed on invertebrates. Prey includes worms, snails, and
insect larvae. Feeding is relatively frequent, and the prey is usually swallowed
whole. Many glossiphoniids suck all the soft parts from their hosts and are best regarded as specialized predators. In laboratory studies Erpobdella punctata
consumed 1.78 tubificids (oligochaete worms) per day and Helobdella stag-nalis
0.57 per day (Cross, 1976).
The bloodsucking leeches attack a variety of hosts. Some, primarily species
of Glossiphonia and Helobdella, feed only on invertebrates, such as snails,
oligochaetes, crustaceans, and insects, but vertebrates are hosts for most species.
Piscicolidae are parasites of both freshwater and marine fish, sharks, and rays. The
glossiphoniids feed on amphibians, turtles, snakes, alligators, and crocodiles.
Species of the cosmopolitan glossiphoniid genus Theromyzon attach to the nasal
membranes of shore and water birds. The aquatic Hirudinidae and the terrestrial
Haemadip-sidae feed primarily on mammals, including humans (Fig. 10-59G).
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 97 of 10
Parasitic leeches are rarely restricted to one host, but they are usually
confined to one class of vertebrates. For example, Placobdella will feed on almost
any species of turtles and even alligators, but they rarely attack amphibians or
mammals. On the other hand, mammals are the preferred hosts of Hirudo.
Furthermore, some species of leeches that are exclusively bloodsuckers as adults
are predacious during juvenile stages.
The mammalian bloodsuckers, suchasHfj| on contacting a thin area of the
host's intej attach the anterior sucker very tightly to M and then slit the skin. The
jaws of HirM about two slices per second. The incisioafl thetized by a substance of
unknown origji pharynx provides continual suction, andthcM tion of hirudin
prevents coagulation of tbf Penetration of the host's tissues is notwdl stood in the
many jawless, proboscis-bearj cies that are bloodsuckers. The proboscis b rigid
when extended, and it is possible! tration is aided by enzymatic action.
Leech digestion is peculiar in a numbd spects. The gut secretes no amylases,
lipases,! dopeptidases. The presence of only ехорерг* perhaps explains the fact
that digestion iJ sucking leeches is so slow. Also character)! the leech gut is a
symbiotic bacterial florae important in nutrition. In both the bloodsi medicinal
leech Hirudo medicinalis and the|a dacious Erpobdella nctoculata. the gut Meters
responsible for a considerable part of digdl they may be significant in the digestion
leeches. The bacterium Pseudomonashin of Hirudo medicinalis breaks down I larweight proteins, fats, and carbohydrate^ the bacterial population increases signihe.
lowing the ingestion of blood by the leech (I 1975). The bacteria may also produce
vital other compounds that are used by theleecJ
Bloodsuckers feed infrequently, but** do, they can consume an enormous
qua blood. Haemadipsa may mgest ten times nsi weight, and Hirudo two to five
times it’s a weight. Following ingestion, water is removed e blood and excreted
through the nephridia. (digestion of the remaining blood cells then epbee very
slowly. These leeches can then tol-t long periods of fasting. Medicinal leeches :en
reported to have gone without food for one years, and since they may require 200
:st a meal, they need not feed more than a year in order to grow.
Lecture # 8. Structure and origin of the excretory system of flat, round and
annelids. Circulatory and respiratory systems nemertine and annelids.
1. Structure and origin of the excretory system of flat, round and annelids.
2. Circulatory and respiratory systems nemertine and annelids.
1.Structure and origin of the excretory system of flat, round and annelids.
Type Flatworms.
The excretory system consists of protonephridia. These are branching canals
ending in so-called flame cells—hollow cells with bundles of constantly moving
cilia.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 98 of 10
Flukes, like other flatworms, have protonephridia, and there is typically a
pair of longitudinal collecting ducts. There may be two anterior, dorsolateral
nephndiopores (in Monogenea) or a single posterior bladder and nephridiopore (in
Trematoda. In the ectoparasites, the protonephridia are probably only
osmoregulatory in function. The function of the protonephridia in en-doparasites is
still uncertain.
Type Nemathelminthes.
Excretion
Protonephridia are absent in all nematodes and) patently disappeared with
the ancestral mere of the class. Some nematodes have no special excretory system,
but many do possess a peculiar system of gland cells, with or without tubules, that
has some excretory function. In the class Adenophorea, which includes most
marine and freshwater nematodes, there is usually one large gland cell, called a
renette gland (Fig. 9-19A), located ventrally in the pseudocoel near the pharynx.
The gland cell is provided with a necklike duct that opens ventrally on the midline
as an excretory pore.
All members of the class Secernentea, which includes many terrestrial
species, have a more specialized tubular system, still composed of only a few cells.
Three long canals are arranged to form an H (Fig. 9-19B). Two are lateral and
extend inside the lateral longitudinal cords. The two lateral cauls are connected by
a single transverse canal, from which a short, common, excretory canal leads to the
excretory pore, located ventrally on the midline. In many nematodes, that part of
each lateral canal anterior to the transverse canal has disappeared, so the system is
shaped like a horseshoe; in others the tubules on one side have been lost, so the
system is asymmetrical.
The excretory gland cell or tubules are known to eliminate foreign
substances, but may have other functions as well. Ammonia is the principal
nitrogenous waste of nematodes and is removed through the body wall and
eliminated from the digestive system along with the indigestible residues.
Type Annelida.
Class Polychaeta.
METANEPHRIDIA
The most common type of excretory organ among coelomate animals is a
metanephridium. In contrast to the blind protonephridial tubule, a metanephridial
tubule opens internally into the coelom. The opening is often funnel-like and
clothed with ciliated perito-num, in which case it is called a nephrostome. In
imsegmented coelomates there may be one nephrite or one to several pairs of
metanephridia; in segmented groups, such as the annelids, the metanephridia are
serially repeated, one pair per segment.
In general, a metanephridium processes coelomic hid. Blood filtrate passes
into the coelom at various sites of filtration, depending on the species. For example, in a mollusk part of the heart wall is the major ate of filtration and is
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 99 of 10
composed of podocytes, cells with finger-like processes that interdigitate [Fig. 1190]. The slits between processes are the sites of titration. Podocytes are found at
the filtration sites of many animals, e.g., the glomeruli of the vertebrate kidney
Coelomic fluid, derived from blood filtrate, passes through the nephrostome into
the ciliated nephridial tubule. Here it becomes modified by selective reab-sorption
and secretion, and the product is finally expelled through the nephridiopore as
urine. The extent of tubular secretion and reabsorption depends in pert on the
environment in which the animal lives, i.e., whether it is an osmoconformer or
osmoregula-tor. The tubule wall is correspondingly specialized and provided with
a vascular backing.
Excretion
Polychaete excretory organs are either protonephridia or metanephridia (Box
10-2). In primitive polychaetes there is one pair of nephridia per segment, but
reduction to few or even one pair for the entire worm has occurred in some
families. The anterior end of the nephridial tubule is located in the coelom of the
segment immediately anterior to that from which the nephridiopore opens (Fig. 102). The tubule penetrates the posterior septum of the segment, extends into the next
segment, where it may be coiled, and then opens to the exterior in the region of the
neuropodium. Both the preseptal portion of the nephridium and the pos-tseptal
tubule are covered by a reflected layer of peritoneum from the septum.
Protonephridia of a type called solenocytes are found in phyllodocids, alciopids,
tomopterids, gly-cerids, nephtyids, and a few others. The solenocytes are always
located at the short preseptal end of the nephridium and are bathed by coelomic
fluid. The solenocyte tubules are very slender and delicate and arise from the
nephridial wall in bunches (Fig. 10-37) Each tubule contains a single flagellum,
and the wall is composed of parallel rods connected by the thin lamellae. The latter
represent the fenestrations through which fluid passes; this arrangement is
characteristic of other types of protonephridia.
All other polychaetes possess metanephridia, in which the preseptal end of
the nephridium possesses an open, ciliated funnel, the nephrostome, instead of
solenocytes. Typical metanephridia are found in the nereids, where the
nephrostome possesses an outer investment of peritoneum and the interior is
heavily ciliated. The postseptal canal, which extends laell next successive segment,
becomes greatly coded form a mass of tubules, which are enclosed J thin, saclike
covering of peritoneal cells. CoiliJ probably an adaptation that increases the игШ
area for tubular secretion or rcabsorption. TkJ phridiopore opens at the base of the
neuropcdJ on the ventral side. The entire lining of thetutJ is ciliated.
The metanephridia of most other polycbl differ only in minor details (Fig.
10-38) bill display various degrees of regional restncwl the more specialized
families. In the fan worn! where only one pair of functional nephndul main, the
two nephridia join at the midline to Л a single median canal, which extends forwrJ
open through a single nephridiopore on tbet. Excretory waste is deposited directly
ouuide, and fouling of the tube is avoided.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 100 of 10
In polychaetes the association of the blood vcs-Klswith the nephridia is
variable. The fan worms HKt the arenicolids lack a well-developed nephridial
blood supply, and the coelomic fluid must be the principal route for waste removal.
In other po-hxhaetes the nephridia are surrounded by a network of vessels. In the
nereids the nephridial blood supply is greater in those species that live in brack-lb
water.
Many polychaetes, particularly nereids, can tolerate low salinities and have
become adapted to life mbrackishsounds and estuaries. The gill (notopo-dullobe)
of Nereis succinea contains cells specially fot absorbing ions. A small number of
species hve in fresh water. The sabellid Manayunkia spe-:wexample, occurs in
enormous numbers in ctrum regions of the Great Lakes, such as around themouth
of the Detroit River. There are a few ter-Шіаі polychaetes, all tropical Indo-Pacific
nereids, which burrow in soil or live in moist litter.
Chloragogen tissue, coelomocytes, and the intestinal wall may play
accessory roles in excretion. Chloragogen tissue is composed of brown or greenish
cells located on the wall of the intestine or on various blood vessels. Chloragogen
tissue, which has been studied much more extensively in earthworms (see p. 316),
is an important center of intermediary metabolism and hemoglobin synthesis.
Class Oligochaeta.
Excretion
The adult oligochaete excretory organs are metanephridia, and typically,
there is one pair per segment except at the extreme anterior and posterior ends. In
the segment following the nephrostome, the tubule is greatly coiled, and in some
species, such as Lumbricus, there are several separate groups of loops or coils.
Before the nephridial tubule opens to the outside, it is sometimes dilated to form a
bladder. The nephridiopores are usually located on the ventrolateral surfaces of
each segment.
In contrast to the majority of oligochaetes, which possess in each segment a
single, typical pair of nephridia called holonephridia, many earthworms of the
families Megascolecidae and Glos-soscolecidae are peculiar in possessing
additional nephridia, which are multiple or branched. Either typical or modified
nephridia may open to the outside through nephridiopores, or they may open into
various parts of the digestive tract, in which case they are termed enteronephric. A
single worm may possess a number of different types of these nephridia, each
being restricted to certain parts of the body.
Earthworms excrete urea, but they are less perfectly ureotelic than are other
terrestrial animals. Although urea is present in the urine of Lumbricus and other
earthworms and although the level of urea depends on the condition of the worm
and the environmental situation, ammonia remains an important excretory product.
Salt and water balance, which is of particular importance in freshwater and
terrestrial environments, is regulated in part by the nephridia (Fig. 10-54B). The
urine of both terrestrial and freshwater species is hypoosmotic, and considerable
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 101 of 10
reabsorption of salts must take place as fluid passes through the nephridial tubule.
Some salts are also actively picked up by the skin.
In the terrestrial earthworms water absorption and loss occur largely through
the skin. Under normal conditions of adequate water supply, the nephridia excrete
a copious hypoosmotic urine. It is not certain whether reabsorption by the ordinary
nephridia is of importance in water conservation, but the enteronephric nephridia
do appear to represent an adaptation for the retention of water. It passing the urine
into the digestive tract, muchl the remaining water can be reabsorbed as it goes
through the intestine. Worms with enteronepmi systems can tolerate much drier
soils or do J have to burrow so deeply during dry periods.
A few aquatic oligochaete species are capabled encystment during
unfavorable environmenrd conditions. The worm secretes a tough, mucca covering
that forms the cyst wall. Some specie form summer cysts for protection against
desicr*! tion; others form winter cysts when thewatertet perature becomes low.
During dry seasons or during the winter, eaii worms migrate to deeper levels of the
soil, dm 3 meters in the case of certain Indian species.Ц moving to deeper levels,
an earthworm often » dergoes a period of quiescence and in drypeJ may lose as
much as 70 per cent of its water.№ ance is restored and activity resumed as soon»
water is again available.
Class Hirudinea.
Excretion
Leech contain 10 to 17 pairs of metanephridia, ill the middle third of the
body, one pair segment. As a result of the coelom ton and the loss of septa in the
leech body, ihndial tubules arc embedded in connective the nephrostomcs project
into the coc-c channels. Each nephrostome opens into a itedcapsule.
In most leeches the cavities of the capsule and idial canal do not connect, and the
two of the nephridium may even have lost ictural connection. Many branching,
intra-ilar canals drain into the nephridial canal, rpens to the outside through the
ventrola-nephndiopore. Secretion into the intracellular iculiisthe initial source of
nephridial fluid, Btfleunne is very hypoosmotic to the blood, in-inung reabsorption
of salts. The nephridia are important organs of osmoregulation (Haupt,
The function of the nephridial capsules is be-Btdtobe the production of
coelomocytes. The coelomocytes are phagocytic and engulf particulate matter, but
the eventual fate of the waste-laden cells is not certain. They may migrate to the
epidermis or to the epithelium of the digestive tract. Particulate waste is also
picked up by botryoidal and vasofibrous tissue of the hirudinid leeches and by
pigmented and coelomic epithelial cells of glos-siphoniids and piscicolids.
2. Circulatory and respiratory systems nemertine and annelids.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 102 of 10
Internal Transport
In most polychaetes there exists a well-developed blood-vascular system, in
which the blood is enclosed within vessels. The basic plan of circulation is
relatively simple. Blood flows anteriorly in a dorsal vessel situated over the
digestive tract; at the anterior of the body, the dorsal vessel is connected to a
ventral vessel by one to several vessels or by a network of vessels passing around
the gut. The ventral vessel carries blood posteriorly beneath the alimentary tract.
In each segment the ventral vessel gives rial one pair of ventral, parapodial
vessels, *һісЦ ply the parapodia, the body wall, and the ni and to several ventral,
intestinal vessels that the gut. The dorsal vessel, intl receives a corresponding pair
of dorsal para| vessels and a dorsal intestinal vessel. The and ventral parapodial
vessels and the dorsal ventral intestinal vessels are interconnected network of
smaller vessels.
There are many variations of this basic tory pattern, and the circulatory
mechani polychaetes are not nearly so uniform as tL scription might suggest. All
polychaetes relv »| varying degrees on the transporting capacity coelomic fluid,
and some have lost the blood-cular system completely.
Although the polychaete system is usually a doled system, in that blood is
restricted to vessels, lithe vessels tend to be relatively thin walled and
ootalwayslined with endothelium (Nakao, 1974). Moreover, the endothelium,
when present, is myoendothelium, and the basal membrane is diked toward the
lumen instead of away from it, ts in vertebrates (Fig. 10-36Л) [Ruppert and Carle,
1983).
The gills are usually provided with afferent and efferent vascular loops
permitting a two-way flow. This is true, for example, of the gills of lugworms
mdthe branchial, notopodial lobes of nereids (Fig. 1Ш|. On the other hand, the
radioles of fan worms, which function in both food gathering and (asexchange,
contain only a single vessel, within whichblood flows tidelike, in and out. In many
po-khaetes, such as glycends, the gills are irrigated nth coelomic fluid and not
blood.
In general, blood is driven by peristaltic waves of contraction that sweep
over the blood vessels, particularly the dorsal vessels. The vessel wall in Уфа. for
example, consists of a single layer of myoepithelial cells that contain striated
myofibrils ananged in a circular direction or in both circular and longitudinal
directions (Boilly and Wissocq, І97Л. Many polychaetes have accessory, heartlike
pumps located in various places within the blood-rascular system.
The blood contains few cells compared with coelomic fluid. In small
polychaetes it is usually colorless, but in larger species and those that burrow in
soft bottoms, the blood contains respiratory pigments dissolved in the plasma. In
fact, within the Polychaeta are found three of the four rcspira-torv pigments of
animals. Hemoglobin is the most common of these pigments, but chlorocruorin is
characteristic of the blood of the serpulid and sa-belhd fan worms and also of the
Flabclligcridac and Ampharetidae. Chlorocruorin is an iron porphyrin like
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 103 of 10
hemoglobin, but the slight difference in side chunsgives it a green rather than a red
color. There I little reason for separating chlorocruorin from pksmahemoglobin,
which it resembles more than ether resembles intracellular hemoglobin. Mage-he
has a blood-vascular system with anuclcatcd corpuscles containing a third ironbearing protein pgment (not a porphyrin) called hemerythrin. Hemerythrin is a
protein similar to hemocyanin rather than a porphyrin, and the molecule of O. is
earned between two iron atoms.
Plasma, or extracellular, hemoglobin and chlorocruorin molecules are
always very large. The piamahemoglobin of Arenicola. for example, con-urns %
heme units. The entire molecule attains a molecular weight of 3,000,000. This
compares with a molecular weight of 60,000 in mammalian hemoglobin, in which
there are four hemes, each attached to a 15,000 molecular weight unit. There are
numerous polychaetes, including Glycera, Cap-itella, and some terebellids, in
which the blood-vascular system is reduced or absent and the coelomic fluid
functions in internal transport. The coelom of these worms contains hemoglobin
located in coelomic corpuscles. Such coelomic hemoglobin, like the corpuscular
hemoglobin of vertebrates, is always a small molecule.
Mangum (1985) believes that Hb packed in red blood cells may be the
primitive condition in animals and has been retained in various animal groups,
including some polychaetes. The more specialized extracellular condition appears
to be related to the lack of capillary beds in most polychaetes. In the larger vessels
through which polychaete blood is pumped, the blood is less viscous with its Hb in
solution than it would be with red blood cells.
There are a number of interesting exceptions to the usual disposition of
respiratory pigments just described. The blood of Serpula contains both hemoglobin and chlorocruorin. Most terebellids and ophelids possess not only
coelomic red corpuscles but also a blood-vascular system with a different
hemoglobin. The two hemoglobins are not alike. The coelomic hemoglobin (Hb)
of Amphitrite has a greater affinity of O, at low oxygen tensions (dissociation
curve to the left) than does the blood-vascular Hb. This difference facilitates the
passage of oxygen from the blood-vascular system to the coelomic fluid, which is
the principal source of oxygen for internal tissues.
In the majority of polychaetes the respiratory pigments function in oxygen
transport, although for only a part of the oxygen consumed. When the blood from
the gills does not become mixed with unoxygenated blood before delivering its
oxygen load to the target tissues, the oxygen affinity of the hemoglobin is
relatively low (oxygen dissociation curve to the right). This is the situation for
polychaetes like Amphitrite and the fan worms (e.g., Sabella), in which the gills
are at the anterior end. In worms with segmental gills, in which the blood from the
gills is mixed with unoxygenated blood en route to the target tissues, the oxygen
affinity of the hemoglobin is high; i.e., the hemoglobin holds on to its oxygen at
relatively low oxygen tensions .
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 104 of 10
Lecture # 9. Structure of larvae and life cycles of flat, round and annelids..
1.
Life cycles of flat, round and annelids.
2.
The structure of the larvae of flat, round and annelids
1. Life cycles of flat, round and annelids
LIF E CYCLES: C LASS TREMATODA
The class Trematoda (digenetic flukes) is the largest group of parasitic
flatworms. Over 6000 q have been described, and new descriptions are continually
being published. There are many species that cause parasitic diseases in man and
domesticated animals.
In contrast to the monogenetic trematodes, life cycles of the digenetic
trematodes involve two to four hosts. The host for the adult is the definitive host,
and the one to three hosts for the numerous developmental stages are termed
intermediate hosts. The adhesive organs are typically two suckers. One sucker,
called the oral sucker, is located around the mouth. The other sucker, the
acetabulum, is located ventrally in middle or posterior end of the body.
Most digenetic trematodes are endoparasirk The definitive hosts include all
groups of vertebrates, and virtually any organ system may be infected. The
intermediate hosts arc largely invertibrates, commonly snails.
The life cycle is complex and will be introduced by a generalized scheme
followed by more specific examples. The egg is enclosed within an oval shell with
a lid, deposited in the gut, and passed to outside with the definitive host's feces. A
snail may ingest an egg containing a miracidium or the ciliiated, free-swimming
miracidium hatched from the egg, or the larval stage may penetrate the snail's
epidermis. It thus comes to inhabit| the hemocoel.
Inside the snail the miracidium, which loses its cilia when it enters the host, begins
a second developmental stage, called a sporocyst. Inside the hollow sporocyst,
germinal cells give rise to a number of embryonic masses. Each mass develops into
another developmental stage, called a redia or daughter sporocyst, which is also a
chambered form. Germinal cells within redia again develop into a number of larvae
called cercariae. The term digenetic refers to this second generation of individuals,
produced asexually.
The cercaria, a fourth developmental stage, possesses a digestive tract, suckers, and
a tail. The cercaria leaves the host and is free swimming. Its second intermediate
host may be an invertebrate (commonly an arthropod) or a vertebrate, in which it
encysts. The encysted stage is called a metacercaria. If the host of the
metacercaria is eaten by the final vertebrate host, the metacercaria escapes from its
cyst, migrates, and develops into the adult form within a characteristic location in
the host.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
Excysting metacercaria
Consumption of infected fish
p. 105 of 10
Encysted mafl| in fish
Figure 7-32 The Chinese liver fluke, Opisthorchis sinensis: A, Dorsal view of adult
worm. B, Life cycle.
Class Cestoda (Cestoidea) Life Cycles
Tapeworms are endoparasites in the guts of vertebrates. Their life cycles
require one, two, or sometimes more intermediate hosts, which are arthropods and
vertebrates. The basic developmental stages are an oncosphere larva, which
hatches free the egg , and a cysticercus or plerocercoid si which is terminal and
develops into an adult though the following few examples illustrate the basic life
cycle patterns of tapeworms, varieties exist.
Diphyllobothrium latum, one of the fish tapeworms, is widely distributed and
parasitic in the gut of many carnivores, including humans. If the egg is deposited
with feces in water, a ciliated, free swimming oncosphere (coracidium) hatches
all an approximately ten-day development. The larva is ingested by certain
copepopod crustaceans. It penetrates the intestinal wall and develops within the
hemocoel into a six-hot stage called a procercoid. When the copepod is ingested
by a variety of freshwater fish the procercoid, like the oncosphere, penetrates the
fish's gut and eventually reaches the striated muscles of the fish to develop into a
plerocercoid stage. The plerocercoid, which looks like an unsegmented tapeworm,
develops into an adult tapeworm when ingested by a definitive host.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 106 of 10
Species of the family Taeniidae are among the best known tapeworms.
Taeniarhynchus saginata, the beef tapeworm, is one of the most common species
in humans, where it lives in the intestine and frequently reaches a length of over 3
meters. Proglottids containing embryonated eggs are eliminated through the anus,
usually with feces. If an infected person defecates in a pasture, the eggs may be
eaten by grazing cattle, sheep, or goats. On hatching in the intermediate host, an
oncosphere larva, bearing three pairs of hooks, bores into the intestinal wall, where
it is picked up by the circulatory system and transported to striated muscle. Here
the larva develops into a cysticercus stage. The cysticercus, sometimes called a
bladder worm, is an oval worm about 10 mm in length, with the scolex
invaginated. If raw or insufficiently cooked beef is ingested by humans, the
cysticercus is freed, the scolex evaginates, and the larva develops into an adult
worm in the gut.
Taenia solium, the pork tapeworm, is also a parasite of humans, but the
intermediate host is the pig and the cysticercus is obtained from pork. Taenia
pisiformis occurs in cats and dogs, with rabbits as the intermediate hosts. This
order (Cyclophyllidea) contains tapeworms that are largely parasitic in birds and
mammals. Vertebrates, insects, mites, annelids, and mollusks serve as intermediate
hosts.
A severe infection of adult tapeworms may cause diarrhea, weight loss, and
reactions to the toxic wastes of the worm. The worms may be eliminated with
drugs. Much more serious is cysticercus infection. Fortunately, the cysticercus
stage of the beef tapeworm will not develop within humans, but this is not the case
for the pork tapeworm, Taenia solium, and for the dog tapeworm, Echinococcus
granulosus. The adult Echinococcus, which lives in the intestine of a dog, is
minute, with only a few proglottids present at any one time. Many different
mammals, including humans, can act as intermediate hosts, although herbivores
are the most important in completing the life cycle. The cysticerci of the pork
tapeworm develop in subcutaneous connective tissue and in the eye, brain, heart,
and other organs. The bladder worm, or hydatid, of Echinococcus develops mostly
in the lung or liver but can develop in many other sites as well. The bladder worms
of both species can be very dangerous when growing in such places as the brain
and can do much damage elsewhere. Hydatid cysts can reach a large size and
contain a great volume of fluid (up to many liters), which if released into the host
can cause severe reactions. Bladder worm cysts can be removed only by surgery.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 107 of 10
Figure. Structure and life cycle of the beef tapeworm, Taeniarhynchus saginatus.
(Adapted from various sources)
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 108 of 10
Phylum Nemathelmintes
Egg
The female nematode lays eggs inside the host, which are then most often
passed out in the host's feces. At this point, the eggs hatch in the external
environment. Cues such as moisture levels or temperature trigger the larva inside
the egg to begin producing enzymes that will dissolve the membrane of the egg. In
response to the right conditions, the larva then also pushes on the weakened
membrane to break through.
Stage 1 and Stage 2 Larvae
Once hatched, the nematode larvae begin to eat bacteria and grow. Some
types of nematode larvae that didn't leave the initial host as an egg, such as
viviparous filarial worms, are transferred to an intermediate host at this time,
usually through the bite of an fly or other arthropod. Or, as in the case of
Strongyloides stercoralis, they might be passed out in the feces as stage 1 larvae.
When the larvae cannot grow anymore because of the size of their cuticle (skin),
they must molt. To molt, they develop a new cuticle under the old one and then
shed the former cuticle. The first molt marks the transition of a larva from stage 1
(L1) to stage 2 (L2).
Stage 3 Larvae
With the notable exceptions of ascarids and pinworms, most nematodes
become infective during the third larval stage (L3) after the second molt. While it
is during this period that they infect the host where they will eventually reproduce,
different nematodes go about it in different ways. In some instances, the host may
become infected by accidentally swallowing the larvae. Other stage 3 larvae, like
those of the hookworm, directly invade the host. Filarial worms, on the other hand,
are again transferred by fly bite from the intermediate host to the final host.
Finally, Trichinela spiralis larvae remain in their initial host, go into dormancy in
the muscles during stage 3, and then infect a new host when it eats the
contaminated meat.
Migration
Once inside the final, or definitive, host, nematode larvae molt another two
times until they develop into immature adults after four larval stages. They also
migrate through the host's body, using the bloodstream or lymphatic system. The
larvae travel from the heart to the lungs, trachea and intestine.
Reproduction
Nemotodes usually require two different sexes to reproduce and, therefore,
develop into both males and females as adults. The males produce sperm in the
testis and deposit them into receptacles in the female. The female holds onto the
sperm until she needs to use them for fertilization. After she has finished
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 109 of 10
fertilization, the eggs develop in her uterus. When they are ready, she then lays
them in the host, using her muscular ovijector. At this point, the life cycle of
nematodes is complete.
Phylum Annelida
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 110 of 10
Reproductive Organs
With the exception of the marine polychaetes, annelids are hermaphrodites:
Each individual has male and female reproductive organs. Still, an annelid cannot
reproduce without a contribution from a mate. Polychaete worms are either male or
female.
Egg Laying
Earthworms, perhaps the most familiar annelids, mate before laying eggs.
Two worms bind themselves to each other while each worm passes a sperm packet
to the other. After mating, the broad, saddle-like band on the worm (called the
clitellum) secretes a mucus sheath that begins to move toward the head of the
worm. As it moves forward, the worm secretes sperm and eggs into the sheath,
which eventually forms an egg cocoon. Terrestrial annelids lay their eggs in the
soil, whereas aquatic annelids deposit or attach their egg cocoon to plants or to the
soil substrate. Marine polychaetes transform into a reproductive stage called an
epitoke before mating. The polychaete's male and female epitokes release sperm
and eggs into the water.
Larval Stage
Marine polychaetes have a free-living larval stage, called a "trochophore."
The trochophore eventually transforms into the adult form.
Adult Habitats
Newly hatched or metamorphosed annelids settle into adult habitats. Most
adult annelids live in the soil. Marine polychaetes live in the soil substrate of their
aquatic habitat. Some marine polychaetes create tubes in the mud, and these
somewhat rigid tubes provide protection. Other parasitic annelids are free-living.
Adult Ecology
Most adult annelids ingest soil, digest organic nutrients and excrete the
inorganic leftovers---sand particles, for example. Some parasitic species such as
leeches, however, feed on other organisms. A few species even prey on other
invertebrates.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 111 of 10
Figure 7-31 Larval types of digenetic flukes: A, Miracidium. B, Sporocyst. C,
Redia. D and E, Cercariae. F, Metacercaria (From the U.S. Naval Medical School
Laboratory Manual.)
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 112 of 10
Figure 7-38 Some stages in the lite cycles of the
fish tapeworm, Diphyiiobothrium latum: A, Ciliated oncosphere. (After Vcrgcer.)
B, Mature- procercoid. (After Brumpt.)
Lecture # 10. General characteristics of the type of Arthropods.
1. Characteristic features of the structure and development. Key members of
their distribution.
2. Features of the organization and development.
3. Most important classes of animals, united in the type of Arthropods.
1. Characteristic features of the structure and development. Key members of
their distribution.
Arthropods are a vast assemblage of animals. At least three quarters of a
million species have been described; this is more than three times the number of all
other animal species combined (see figure inside cover). The tremendous adaptive
diversity of arthropods has enabled them to survive in virtually every habitat; they
are perhaps the most successful of all the invaders of the terrestrial habitat.
Arthropods represent the culmination of evolutionary development in the
protostomes. They arose either from primitive stocks of polychaetes or from
ancestors common to both, and the relationship between arthropods and annelids is
displayed in several ways.
1 Arthropods, like annelids, are metameric. Metamerism is evident in the embryonic development of all arthropods and is a conspicuous feature of many adults,
especially the more primitive species. Within many arthropod groups there has
been a tendency for metamerism to become reduced. In such forms as mites, for
example, it has almost disappeared. Loss of metamerism has occurred in three
ways. Segment become lost, segments have become fused together, and segmental
structures, such as ц ages, have become structurally and functio. ferentiated from
their counterparts on segments. Different structures having the same segmental
origin arc said to be serially homologous. Thus, the second antennae of a crab are
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 113 of 10
serially homologous to the chclipeds (claws), for evolved from originally similar
segmental appendages.
2 In the primitive condition each arthropod segment bears a pair of appendages.
This same condition is displayed by the polychaetes, in which each metamere bears
a parapodia. However, the homology between pi podia and arthropod appendages
is uncertain.
3 The nervous systems in both groups are constructed on the same basic plan. In
both a dorsal anterior brain is followed by a ventral nerve cod containing
ganglionic swellings in each segment.
4 The embryonic development of a few pods still displays holoblastic determinate
dermage, with the mesoderm in these forms arising from the 4d blastomere.
Exoskeleton
Although arthropods display these annelidan characteristics, they have
undergone a great many profound and distinctive changes in the course of their
evolution. The distinguishing feature of arthropods, and one to which many other
changes are related, is the chitinous exoskeleton, or cuticle, that covers the entire
body. Movement is made possible by the division of the cuticle into separate
plates. Primitively, these plates are confined to segments, and the plate of one
segment is connected to the plate of the adjoining segment by means of an articular
membrane, a region in which the cuticle is very thin and flexible. Basically, the
cuticle of each segment is divided into four primary plates—a dorsal tergum,
two lateral pleura, and a ventral sternum (Fig. 1). This pattern has frequently
disappeared because of either secondary fusion or subdivision.
Fig 1 , Cross section.
The cuticular skeleton of the appendages, like that of the body, has been
divided into tubelike segments, or sections, connected to one another by articular
membranes, thus creating a joint at each junction. Such joints enable the segments
of the appendages, as well as those of the body, to move (hence the name of the
phylum, Arthropoda— jointed feet). In most arthropods the articular membrane
between body segments is folded beneath the anterior segment. In| arthropods the
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 114 of 10
additional development condyles and sockets is suggestive of ventral skeletal
structures.
In addition to the external skeleton, then in also been the development of
what is called the endoskelcton. This may be ail of the procuticle that produces
inner apodemes, to which the muscles, or it may involve the sclerotizing internal
tissue, forming free plates for muscle attachment within the body.
The arthropod skeleton is secreted by the derlying layer of integumentary epithet
known as the hypodermis. It is composed of outer epicutiece and a much thicker
procuticle (Fig 2).
The epicuticle is composed of proteins in many arthropods, wax. The fully
developed cuticle consists of an outer exocuticle and endocuticle. Both layers are
composed of derm and protein bound together to form a comlex of coprotein, but
the exocuticle in addition has been tanned, i.e., with the participation of phenols
molecular structure has been further stall the formation of additional cross
linkages. Exocuticke is absent at joints and along lines where the skeleton will
rupture during molting. In arthropods the procuticle is also impregnated mineral
salts. This is particularly true for the Crustacea, in which calcium carbonate and
calcic phosphate deposition takes place in the proc. Where the exoskeleton lacks a
waxy epicuticle at ЙМ is a relatively permeable covering and alklhtpassageofga.es and water. The cuticle is pcillv penetrated by fine pore canals,
which htm as ducts for the passage of secretions of ■dedyinggland cells.
Theanhropod cuticle is not restricted entirely Ifcotenorof the body. The
hypodcrmis devel-Щ horn the embryonic surface ectoderm, and all ildiogs of the
original layer, such as the- fore-khmdgut, which develop from the stomodeum llthe
proctodeum, thus are lined with cuticle fcB-MI. Other such ectodermal derivatives
input tracheal (respiratory! tubes of insects, ttpods, diplopods, and some arachnids;
the Wlungs of scorpions and spiders; and parts of Ifcreproductive systems of some
groups. All of bintemal cuticular linings are also shed at the it of molting.
I ftecolorof arthropods commonly results from bdtposition of brown, yellow,
orange, and red ^pigments within the cuticle. However, іг-fctot greens, purples,
and other colors result
bfestriationsof the epieutiele, which caustic refaction and give the appearance of
color. Ы,body coloration docs not originate directly cuticle but instead is produced
by subcuti-thromatophores or is caused by blood and tis-ipigments, which arc
visible through a thin, (cuticle.
Despite its locomotor and supporting advantages, an external skeleton poses
problems for a growing animal. The solution evolved by the arthropods has been
the periodic shedding of the skeleton, a process called molting or ecdysis.
Before the old skeleton is shed, the epidermal layer (hypodermis) secretes
proenzymes (inactive enzymes) at the base of the skeleton. The hypodermis now
detaches from the skeleton, a process referred to as apolysis, and secretes a new
epieutiele or at least its outer cuticulin layer (Fig. 12-4B). The proenzymes
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 115 of 10
secreted earlier—chitinase and protease—become activated and digest the untanned endocuticle (Fig. 12-4C). The products of digestion are reabsorbed through
the new cuticulin envelope. With the erosion of the old endocuticle, the
hypodermis secretes new procuticle.
At the ultrastructural level there are only three components to the skeleton. The
outer cuticulin envelope and chitin fibers, the latter forming most of the arthropod
skeleton, are laid down by plasma membrane plaques of the hypodermis (Fig. 124E). These two skeletal components are separated by the third component,
proteins, which are deposited by exocytosis (Locke, 1984).
At this point the animal is encased within both an old and a new skeleton (Fig. 124D). The old skeleton now splits along certain predetermined lines and the animal
pulls out of the old encase- ment. The new skeleton is soft and commonly wrinkled
and is stretched to accommodate the increased size of the animal. Stretching is
brought about by blood pressure, facilitated by the uptake of water or air by the
animal. Hardening of the cuticle results from tanning of the protein and from
stretching.
Additional procuticle may be added following ccdysis, and in some arthropods,
such as insects, additions are made to the epicuticle by secretions through the pore
canals. The final surface of the epicuticle is often formed by a cement layer.
Sensory structures and muscle connections pose special problems for the molting
process. Sensory structures, such as hairs, are laid down bena old skeleton, usually
horizontally against 4 skeleton. The dendrite may retain connectia the old hair until
broken at ecdysis.
Muscles are attached to the exoskcletonl crotubules in specialized epidermal cells.
Tj crotubules are anchored to an internal fold exoskeleton containing a fiber that
runs all tl to the epicuticle (Fig. 12-5). The fiber is J gested during the molting
process and mainl connection between the old and new site until severed at
ecdysis.
The stages between molts are known asii and the length of the instars becomes
longer »mes older. Some arthropods, such as almost crabs, continue to molt
through-Ik Other arthropods, such as insects and lm more or less fixed numbers of
instars, ring attained with sexual maturity. Oghanarthropod is measurably larger
and llowingecdysis (Fig. 12 -6), growth is ac-nuous, as in most other animals. Proper organic compounds arc synthesized lintermolt period, replacing fluids taken
ing is under hormonal control. Ecdvsonc, Iky certain endocrine glands (tor
example, iracic glands in insects), is circulated by ■Beam acts directly on the
epidermal cells. The production of ecdysone is in turn regulated by other
hormones. Although most studied and best understood in insects and crustaceans,
ecdysone controls molting in all arthropods. Molting physiology will be described
in some detail for crustaceans
Movement and Musculature
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 116 of 10
As movement in arthropods has become restricted to flexion between plates
and cylinders of the cuticle, a related change has taken place in the nature of the
body musculature. In annelids the muscles take the form of longitudinal and
circular sheathlike layers of fibers lying beneath the epidermis. Contraction of the
two layers exerts force on the coelomic fluid, which then functions as a hydrostatic
skeleton. In arthropods, on the other hand, these muscular cylinders have become
broken up into striated muscle bundles, which are attached to the inner surface of
the skeletal system (Figs. 12-1B and 12-2).
The muscles are attached to the inner side of the exoskeleton by specialized
hypodermal cells (Fig. 12-5). Flexion and extension between plates are effected by
the contraction of these muscles, with muscles and cuticle acting together as a
lever system. This со functioning of the muscular system and skeletal system to
bring about locomotion is similar to that in vertebrates. Extension, particularly of
the appendages, is accomplished, in part or entirely, by an increase in blood
pressure.
Arthropods employ as their chief means of locomotion jointed appendages, which
act either as paddles in aquatic species or as legs in terrestrial groups. Our
knowledge of arthropod locomotion, especially locomotion on land, results largely
from the extensive studies of Manton (1978). In contrast to the parapodia of
polychaetes, the locomotor appendages of arthropods tend to be more slender,
longer, and located more ventrally. Despite the more ventral position of the legs,
the body usually sags between the limbs (Fig. 12-7A). In the cycle of movement of
a particular leg, the effective step, or stroke, during which the end of the leg is in
contact with the substratum, is closer to the body than the recovery stroke, when
the leg is lifted and swung forward (Fig. 12-7B to £). Among the several factors
determining speed of movement, the length of stride is of obvious importance, and
stride lop increases with the length of the leg. The ргоЫя of mechanical
interference are decreased by duction in the number of legs to five, four, or tl pairs
and by differences in leg length and therij tive placement of the leg tip. In
arthropods thi have retained a large number of legs, suchasel tipedes, the fields of
movement of individual! overlap those of other legs (Fig. 12-7BL Ford* animals
the difference in proximity of the lepl the body during the effective and recovery
stroke prevents mechanical interference.
The arthropodan gait involves a wave of lot movement, in which a posterior leg
isputdfl^ just before or a little after the anterior leg is lil The movements of legs on
opposite sides ofj body alternate with one another; i.e., one pair is moving through
its effective strokewhilei| mate is making a recovery stroke. AlternateЦ movement
tends to induce body undulation.! tendency is counteracted by increased bodyi\ ity,
such as the fused leg-bearing segmentsd form the thorax and cephalothoraxof
insccts,H crustaceans, and arachnids.
An exoskeleton makes a highly efficient! motor-skeletal system for animals that
are onhil few centimeters long. It provides protection red dition to support, and
there is a large surfaced for the attachment of muscles The tubular ad struction
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 117 of 10
resists bending. However, the walla buckle on impact if there is insufficient skekd
material, just as you cannot bend a cvlindricaleJ but you can buckle one wall by
kicking it. Тһим| exoskeleton imposes limits on the maximum! of arthropods. The
weight of a large animalandtkj resulting stress produced when moving wouldd
quire heavy skeletal walls. But when theartbT molted, the soft, new skeleton would
collaj under the animal's weight before hardeningoj occur. Significantly, the largest
arthropods livtil the sea, where the aeiuatie medium providesraT more support than
air.
In contrast to the condition in vcrtebrates,d arthropod muscle contains relatively
few fibend is innervated by only a small number of neun Many axon terminals arc
provided to one mud fiber (Fig. 12-8), and one neuron may supply m than one
muscle. Moreover, several typesofm) neurons—phasic (fast) neurons, tonic
islowldj rons, and inhibitory neurons may supplvasindj muscle. The terms phasic
and tonic, or fasti slow, refer not to the speed of transmissionbtnj the nature of the
muscle response. The impulK phasic motor neurons produce rapid but briefco*
trains, which are often involved in rapid movc-■ats. The impulses of tonic motor
neurons pro-in slow, powerful, prolonged contractions, id)are involved in postural
activities and slow anements. The impulses of inhibitory neurons i»i contractions.
Tie neuromuscular system may be further implicated, as in at least the crustaceans,
by the ttrentiationofthe muscle fibers into phasic and Wtypes,each having a
distinctive ultrastructure ffldphvsiclogy. Some muscles arc entirely phasic, ■rare
entirely tonic, and some are mixed. Phasic Iworneurons innervate only phasic
muscle fibers; \m motor neurons innervate both phasic and Lib or, in some
instances, only tome fibers.
la vertebrates graded responses depend in large I pen die number of motor units
contracting. Ar-^muscles are not organized as motor units, awever. and graded
responses depend on the type (muscle fibers contracting, the type ot neuron
Wandthe interaction of different types of ncu-■ №example, two different extensor
muscles Kinnervated by the same motor fiber in a crayfish dw.but the two muscles
function independently Leeach is innervated by separate inhibitory ■ras.
The organization of arthropod ganglia is like Moi annelids and mollusks described
on page 21.Giant fiber systems are frequently well devcl-ajd,and "command"
systems have been identi-U.Arthropod neural networks and neuromuscu-isystems
have been best studied in crustaceans. 'Bltsted students should consult Kennedy et
al
(1969), Atwood (1973), and Atwood and Sandeman (1982).
Coelom and Blood-Vascular System
The well-developed, metameric coelom characteristic of the annelids has
undergone drastic reduction in the arthropods and is represented by only the cavity
of the gonads and in certain arthropods by the excretory organs. The change is
probably related to the shift from a fluid internal skeleton to a solid external
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 118 of 10
skeleton. The other spaces of the arthropod body do not constitute a true coelom
but rather a hemocoel—that is, merely spaces in the tissue filled with blood.
Although derived from the annelids, the arthropod blood-vascular system is an
open one. The dorsal vessel of annelids, which is contractile and the chief center
for blood propulsion, may be homologous to the arthropod heart. The heart varies
in position and length in different arthropodan groups, but in all of them the heart
is a muscular tube perforated by pairs of lateral openings called ostia (Fig. 12-1A).
Systole (contraction) results from the contraction of heart wall muscles, and
diastole (expansion and filling) from suspensory elastic fibers and in some species
from the contraction of suspensory muscles. The ostia enable the blood to flow into
the heart during diastole from the large, surrounding sinus known as the
pericardium. However, in arthropods the pericardium does not derive from the
coelom, as in mollusks and vertebrates, but is a part of the hemocoel. After leaving
the heart, blood is pumped out to the body tissues through arteries and is
eventually dumped into sinuses (collectively the hemocoel) in which it bathes the
tissues directly. The blood then returns by various routes to the pericardial sinus.
The blood of arthropods contains several types of cells and in some species the
respiratory pigment hemocyanin or, less commonly, hemoglobin. As in mollusks,
arthropod hemocyanin is a large molecule dissolved in the plasma; however, the
structure of arthropod hemocyanin indicates that it evolved independently from
that of mollusks (see reviews by Mangum, 1985; Linzen et al, 1985).
Arthropods possess two types of excretory organs. Malpighian tubules are blind
tubules that lie within the hemocoel (blood-filled spaces) and open into the gut.
Wastes pass from the blood into the tubules and then into the gut, where they are
eliminated through the anus along with fecal material. Malpighian tubules are
found in centipedes, millipedes, insects, and arachnids and represent an organ
system that evolved independently within these groups or their arthropod
ancestors.
The other type of arthropod excretory organ is paired blind saccules that open by
ducts to the outside of the body adjacent to an appendage (Fig. 129A). The
excretory organ takes the name of the appendage with which it is associated—
coxal glands, maxillary glands, etc. Since the saccule is derived embryonically
from the coelom, the tubule may represent an old metanephridium that originally
drained the coelom. Typically, the saccule wall is composed of podocytes (Fig. 129B) and is the site of filtration from the surrounding blood. Parts of the tubule may
be modified for selective reabsorption and secretion. Although such paired
excretory organs may be derived from nephridia, no living arthropod has more than
a few such saccules.
Digestive Tract
The arthropod gut differs from that of most other animals in having large
stomodcal and proctotj regions (Fig. 12-1A). The derivatives of thai todermal
portions are lined with chitin and соиі tute the foregut and hindgut. The
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 119 of 10
intervenioil gion, derived from endodcrm, forms the midpt The foregut is chiefly
concerned with ingest* trituration, and storage of food; its parts are J iously
modified for these functions, depending the diet and mode of feeding. The midgut
ill site of enzyme production, digestion, andafcJ tion; however, in some arthropods
enzymes! passed forward and digestion begins in the fore Very commonly the
surface area of the midgat I increased by outpocketings, forming pouchaJ large
digestive glands. The hindgut functionsaB absorption of water and the formation of
feces.
Brain
There is a high degree of cephalization inaitJ pods. The increase in brain size is
correlated*! well-developed sense organs, such as eyes audi tennae, and many
arthropod groups display » plex behavioral patterns. The arthropod Щ consists of
three major regions—an anterior J tocerebrum, a median deutoeerebrum, and ltd
terior tritocercbrum (Fig. 12 - 10A|. The neml from the eyes enter the
protocerebrum,whichdJ tains one to three pairs of optic centers |мі piles). The optic
and other neuropiles of the (J tocerebrum function m integrating photorecal and
movement and are probably the centers fori initiation of complex behavior.
The deutoeerebrum receives the anted nerves (first antennae in crustaceans) and
сопи their association centers. Antennae are lackafl the chelicerates (scorpions,
spiders, mites),anil these arthropods there is a corresponding absd of the
deutoeerebrum (Fig. 12-1CLB).
The third brain region, the tritocerebrum,! rise to nerves that innervate the labium
ilowefJ the digestive tract (stomatogastric nervafl chelicerae (claws) of
chelicerates, and the seal antennae of crustaceans. The commissure ofl
tritocerebrum is postoral, i.e., behind the fond
A debate centers on the extent to whichtH thropod head is a segmented structure.
Mostd ogists agree that the tritocerebrum is a scgmaJT ganglion that has shitted
anteriorly. Its pad commissure alone is good evidence of suchttl gin. Anderson's
review of annelid and arthropod serology (19731 supports the belief that the bdtof
all arthropods contain two or three preoral qnentsand that the antennae are
segmental ap-|0dlgcs. A resume of older ideas on the subject is dinedby Bullock
and Horridge (1965).
Sense Organs
Ііямгу receptors of arthropods are usually as-Bated with some modification of the
chitinous ■skeleton, which otherwise would act as a Ьаг-blhe detection of external
stimuli. A modified the exoskeleton for the reception of cn-nementalinformation
other than light is called .ШІҺші. Sensilla have various shapes, dependijMtherypeof signals they are designed to mon-Elhemost common form is a hair,
bustle, or |ktthere are also sensilla in the form ot pegs, kndsli's. Each sensillum is
composed of one inoresensory neurons plus a number of cells ^produce the
housing of the apparatus. Some ВВІаcontain a single type of receptor neuron;
etasencompass a number of types. For example, ■insect"taste" hairs contain both
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 120 of 10
ehemorecep-■mdmechanorcceptors. Thus, sensilla cannot inys be classified by
function. In general, those мшщchemoreceptors have perforated walls I Ц
Mechanoreceptors are stimulated bv тешем of the sensilla, as in the ease ot hairs
fltl3-7D|, or by changes in tension on the ex ,*ktoii,asinthe case of slit sensilla
(Fig. U 91. Ik concentrations of sensilla over the arthropod (Id)limply reflect points
of most likely contact menuls to be monitored. Arthropods also possess
proprioreceptors attached to the inside of the integument or to muscles.
Most arthropods have eyes, but the eyes vary greatly in complexity. Some are
simple and have only a few photoreceptors. Others are large, with thousands of
retinal cells, and can form a crude image. In all arthropods the skeleton contributes
the transparent lens-cornea to the eye.
Insects and many crustaceans, such as crabs and shrimp, have compound eyes
composed of many long, cylindrical units, each possessing all the elements for
light reception. Each unit, or omma-tidium, is covered at its outer end by a
translucent cornea derived from the skeletal cuticle (Fig. 12-11). The cornea
functions as a lens. The external surface of the cornea, called a facet, is usually
hexagonal or sometimes square (as in crayfish and lobsters). Behind the cornea, the
ommatidium contains a long, cylindrical or tapered element called the crystalline
cone, which functions as a second lens.
Lecture #11. External morphology of crustacean.
1. Features like crustaceans protoaquatic arthropods.
2. Types of development, larval stages.
3. Characteristic features of the structure and development. Key members of
their distribution.
4. Features of the organization and development of higher crustaceans. Marine,
freshwater and terrestrial isopods. Fishing crustaceans, their fishery.
Lecture #12. External and internal structure Invertebrates.
1. Features of the organization as a spider to land in the majority of prey
helitserovyh.
2. Dismemberment of the body arachnids.
3. Internal structure of arachnids.
Lecture #13. Features of the external structure of insects. Head and its
appendages.
1. Features of the organization insects as arthropods adapted to life on land, in
the air.
2. General morphological and physiological characteristics of insects.
Lecture #14. Reproduction and development of insects.
1. Insects with incomplete metamorphosis.
2. Insects with complete metamorphosis.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 121 of 10
Lecture #15. External morphology type Shellfish.
1. Avilable type of shellfish.
2. General characteristics Class Gastropods.
3. General characteristics Class lamellibranch clams.
3 LABORATORY CLASSES
1.
2.
3.
4.
Laboratory #1. Basic techniques of microscopy. Sunfish and pod structure.
Principles of living objects. Retainers, appliances cooking.
Methods for the preparation of dyes.
Avilable pod. Reproduction.
Avilable sunfish.
Purpose of the lesson:
1.Principles of living objects. Retainers, appliances cooking. Methods for the
preparation of dyes.
NOTES FOR GUIDANCE IN MAKING PERMANENT PREPARATIONS.
Only very simple directions are here given, such as will serve to aid students
who have had no experience in preparing objects for microscopic examination to
make preparations when this is desirable for proper laboratory study Those who
desire to prepare material for serial sections, or who wish to make whole mounts of
delicate material, are referied to Lee's "Micro-tomist's Vadc Mecum."
The steps taken in preparing total mounts include:
1. Fixing, or killing.
2. Washing.
3. Dehydrating and staining.
4. Clearing
5. Mounting.
Fixing.—This is necessary to keep the cells and tissues as nearly as possible in
their natural position, shape, and structure, and in order that the protoplasm
composing them may be kept in condition to stain satisfactorily.
In selecting a fixing agent remermVr:
1. If the material is highly irritable and contractile, it will have to be killed
practically instantly with hot solutions, or be previously narcotized.
2. If there is much lime, an agent that contains much ac id should not be used, as
the lime will be dissolved and the bubbles of gas are likely to tear or distort
tissues
3 Where rapid fixation is desirable, as in expanded hydroids and the like,
sublimate-acetic (hot) is preferable Where the tissue, or the animal, is not specially
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 122 of 10
muscular, or liable to contraction, any of the fluids can be used. The time objects
should be left in the killing solution varies, approximately, dnectly as
GUIDANCE IN MAKING PERMANENT PREPARATIONS.
their size. Three minutes will suffice for killing hydroids in sublimate-acetic.
Washing.—All objects must be thoroughly washed, after using most killing
agents. This may be done with repeated changes of fresh water or with alcohol,
beginning with a low grade and gradually working up to 70 percent. With most
small objects alcohol is preferable, but if the object is large this is too expensive.
In case a fixing agent is used which is an alcoholic solution, wash out in the same
grade of alcohol used in making the fixing agent
topic, , methodical recommendations, questions for self-protection and
laboratory classes Dehydrating and Staining.—From water, all objects should be
placed successively in 35 percent, 50 percent, and 70 percent alcohol, five to
fifteen minutes in each for small objects. In subsequent changes from one grade to
another allow about the same time All tissues killed in a corrosive sublimate mixture should now be treated with a weak solution of lodin, to dissolve the corrosive
sublimate that still remains, and thus prevent the later formation of crystals of that
substance. Such crystals would not appear immediately, but ever increasingly, as
the preparation is kept. Put a few drops of iodin into the 70 percent alcohol
containing the object, leave a few minutes, and, if the yellow color caused by the
iodin has disappeared, turn off the alcohol and use more 70 percent alcohol with
iodin, as before The bleaching indicates that some corrosive sublimate remains.
Repeat until the yellow color does not fade. Then wash in clear 70 percent alcohol.
At this point either staining, or preparation for so doing, begins.
In case the stain you wish to use is a 70 percent alcoholic solution, it may be
used immediately. Otherwise, the object must be run through the grades of alcohol,
up or down as the case may be, to .that medium in which the stain to be used is
dissolved If an aqueous stain such as alum-carmine is to be used, pass through 50
percent and 35 percent alcohol to water. If a 95 percent alcoholic stain is to be
used, pass through SO percent and 95 percent alcohol.
CLEARING AND MOUNTING.
The time an object should be treated with stain varies with the stain and the size
of the object. Alum-carmine should be used from six to twenty hours, according to
circumstances. Borax-carmine should be used from five minutes to half an hour
Aceto-carmine, used for killing and staining, acts very rapidly Delafield's
hematoxylin (a dark wine-colored solution in water) requires ten minutes to half an
hour. In all these cases, examination of the objects themselves is the only means of
deciding when staining is sufficient. It is usually best to slightly over-stain and
then to bleach out, as certain parts of the protoplasmic structure will retain the stain
better than others and thus better differentiation will be secured. After staining,
bring the tissues gradually into 70 percent alcohol, and then treat with acidulated
alcohol to remove excess of stain. After this, every trace of the acid must be
removed by washing in clean alcohol, or the tissues will continue to bleach after
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 123 of 10
they are mounted. The specimen is now ready for final dehydration. In damp
climates, as at the seashore, your stronger alcohols must be kept closely covered all
of the time or they will take water from the atmosphere and be unfit for the
purpose. Run through SO percent, 95 percent, and 100 percent alcohol, thus
completing dehydration Every trace of water must be removed and then kept out.
Clearing and Mounting.—From absolute alcohol, place objects in some clearing
fluid (clove oil, cedar oil, or xylol) and leave till they have a clear, translucent
appearance, after which place on a clean slide, with come Canada balsam or
dammar, and cover with a cover-glass.
If the object turns cloudy or milky when placed in the cleaning fluid, it is evidence
that all of the water has not been removed, and it should be returned to absolute
alcohol for complete dehydration Tissues left in the clove oil or xylol for any great
length of time will become hard and brittle. In case tissues in the process of
preparation must necessarily be left untreated for several days, they should be left
in a 70 percent or 80 percent alcoholic medium 12
GUIDANCE IN MAKING PERMANENT PREPARATIONS.
Sectioned Material.—In a few cases sectioned material may be distributed to the
class. Be sure that the slide on which you intend mounting the sections is
tlioroughly clean. Remove any greasy substance with 95 percent alcohol. On a
cleaned slide, smear a very little albumen with your finger-tip and remove all
except the thinnest film. Now place the sections on the albumen over an area the
size of the cover-glass to be used, and press them down fiat with the tip of a clean,
dry finger.1 Warm the slide over an alcohol lamp very carefully until the paraffin in
which the sections are embedded is just melted While the paraffin is still melted
treat it with хзг1о1 (a jar containing xylol for this purpose is desirable). This will
dissolve the paraffin and leave the sections alone adhering to the slide. When the
paraffin is completely dissolved (this will take but a few seconds), drain off the
xylol, apply a drop of balsam, and cover as in total mounts. The preparation is now
ready for use, and is permanent, but must be handled carefully while fresh.
Application of above directions in the case of a hydroid: Hot corrosive, fifteen
seconds. Cold corrosive, five minutes.
Water or alcohol, four changes, three or four minutes each.
Thirty-five percent, 50 percent, and 70 percent alcohol,
five minutes each. Seventy percent alcohol plus iodin, as in directions
above.
One-half of your material may now be placed in borax-carmine. Leave the material
in this till objects have taken on a good color. (Ask an instructor about this ) When
sufficiently stained, put into acidulated alcohol till the color assumes a brilliant
appearance, but do not allow it to fade too far. Wash in 70 percent and then tun
through SO pet cent, 95 percent, and 100 percent alcohol, five minutes in each,
thence into clove oil, or cedar oil, keeping all reagents carefully covered, and leave
till the object is thoroughly penetrated. This latter piocess may take f i v e to ten
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 124 of 10
minutes If, on putting youi objects into the clearing medium, the latter exhibits a
nnlky-white appearance, the material is not sufficiently dehydrated, and must be
returned to 100 percent alcohol
After clearing is completed, put the object on a clean shdo with a little balsam
and cover.
The material not treated with borax-carmine may be run back through 50 jiercent
and 35 percent alcohols to water, to which a few chops of hematoxylin have been
added, or put from water into alum-carmine The former stain, if dense, should not
require over twenty to thirty minutes, but objects must be left in alum-carmine ten
to twenty hours When a good color is obtained, run the material through the grades
of alcohol, from the lowest to the highest (five minutes in each), and mount as in
the case of the borax-carmine objects.
Objects stained in alum-carmine will probably not overstain, but excess of
hematoxylin should be extracted with acidulated alcohol when the 70 percent giade
is reached, after which it is very essential that all of the acid be removed by
repeated changes of 70 percent alcohol. Otherwise the objects will fade.
2.Avilable of Radiolaria and Heliozoa. Reproduction.
RAD IO LAR IA Heliozoa
Among the most beautiful of the protozoa arc-members of three classes,
collectively called radiolanans. They are entirely marine and primarily planktonic.
Radiolarians are relatively large- protozoa; some species are several millimeters in
diameter, and some colonial forms attain a length of up to 20 cm (Collozoum).
Like heliozoans, the bodies of radiolarians are usually spherical and divided into
inner and outer parts (Fig. 2-17). The inner region, which contains one to many
nuclei, is bounded by a central capsule with a membranous wall. The capsule
membrane is perforated by openings, which may be evenly distributed or restricted
to three pores located at specific sites in the membrane. The perforations allow the
cytoplasm of the central capsule (or intracapsular cytoplasm) to be continuous with
the cytoplasm of the outer division of the body. This extracapsular cytoplasm
forms a broad cortex, called the ca-lymma, that surrounds the central capsule.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 125 of 10
Figure 2-17 Acanthometra, a radiolarian with a skeleton of radiating strontium
sulfate rods. (From Farmer, J. N, 1980: The Protozoa. С. V. Mosby Co., St. Louis,
p. 353.)
In many species the calymma contains large numbers of symbiotic
dinoflagellates (zooxanthel-lae) in a nonflagellate stage as well as many vacuoles,
which give the cytoplasm a frothy appearance. As in corals and some other marine
animals, the excess organic photosynthate produced by the zooxanthellae is
utilized by the radiolarian host as an accessory food source.
The pseudopodia are axopods and radiate from the surface of the body. Their
axes of microtubules originate within the central capsule and extend through the
calymma.
A skeleton is almost always present in radiolarians and is usually siliceous,
but in the class Acantharia it is composed of strontium sulfate. Several types of
skeletal arrangements occur. One type has a radiating structure, in which the
skeleton is composed of long spines or needles that radiate from the center of the
central capsule and extend beyond the outer surface of the body (Fig. 2-17). The
points where the skeletal rods leave the body surface are surrounded by contractile
fibrils (as in Acantharia). The action of these fibrils can cause the calymma to be
expanded. A second type of skeleton is constructed in the form of a lattice sphere,
which is often ornamented with barbs and spines.
The planktonic radiolarians display a distinct vertical stratification from the
ocean surface down to 4600-meter depths. The great numbers in which planktonic
foraminiferans and radiolarians occur arc indicated by the fact that their shells,
sinking to the bottom at death, form a primary constituent of many ocean bottom
sediments.
Locomotion
Flowing ameboid movement is limited to those Sarcodina that possess
lobopods or filopods and has been most studied in the naked amebas. In these
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 126 of 10
animals locomotion may involve a single large, tubular lobopod or several small
ones with caps of hyaline protoplasm at the tips.
The pseudopodia of most heliozoans and radiolarians are food-capturing
rather than locomotor organelles. However, radiolarians are able to move vertically
in the water by extending or contracting the calymma and axopods, by increasing
or decreasing the vacuolated condition of the calymma, and by the presence of
endoplasmic oil droplets.
Nutrition
The Sarcodina are entirely heterotrophic. Their food consists of all types of
small organisms: bacteria, algae, diatoms, protozoans, and even small multicellular
animals such as rotifers, copepod larvae, and nematodes. The prey is captured and
engulfed by means of the pseudopodia.
In the amebas, pseudopodia extend around the prey, eventually enveloping it
completely with cytoplasm, or the body surface invaginates to form a food cup.
The enclosing of the captured organism by cytoplasm results in the formation of a
food vacuole within the ameba.
In foraminiferans, heliozoans, and radiolarians the numerous radiating
pseudopodia (chiefly reticulopods or axopods) act primarily as traps in the capture
of prey. Any organism that comes in contact with the pseudopodia becomes
fastened to the granular, adhesive surface of these organelles. A granular mucoid
film is especially evident on the surface of foraminiferan reticulopods and quickly
coats the surface of captured prey. This film contains lysosomes, and their
proteolytic secretions aid in paralyzing the prey and initiate digestion even during
capture. The long spines of many planktonic forams are also covered with the mucoid film and arc important as a food-trapping surface rather than as a flotation
mechanism. They are able to capture fairly large prey, such as small crustaceans.
In all three groups food particles are enclosed in food vacuoles and drawn toward
the interior of the body. The axial rods of heliozoans may contract, drawing the
prey into the ectoplasmic cortex, or the axopods may liquefy and surround the
food, forming a vacuole at the site of capture. The vacuole will then be moved
inward.
Digestion occurs in the cortex of heliozoans and the calymma of
radiolarians. In acantharian radiolarians, digestion of at least small particles takes
place largely inside the central capsule. Where an enveloping skeletal sphere is
present, the food passes through the openings for the pseudopodia. In
foraminiferans food is initially digested outside the shell, and then digestion is
completed within small food vacuoles within the shell.
Egestion can take place at any point on the surface of the body, and in the
actively moving amebas, wastes are usually emitted at the posterior, as the animal
crawls about.
Reproduction and Life Cycle
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 127 of 10
Asexual reproduction in most amebas, heliozoans, and radiolarians is by
binary fission (Fig. 2-19Л). In amebas with a soft shell, the shell divides into two
parts, and each daughter cell forms a new half.
Laboratory #2 Animal kingdom. Subkingdom - celled animals Protozoa. Species
diversity and structure features naked amoebae.
1. Avilable naked amoebae.
2. Features of the development of naked amoebae.
SARCODINA.
AMOEBA PROTEUS.
Amoebae are usually easily discernible under the low power of the microscope
as irregular, semi-transparent, granular bodies. Find a specimen in the material
provided, which is known to contain amoebae, and determine the following points:
1. With the high power observe the peculiar method of locomotion, the constant
but slow change in the shape of the body by means of projections, pseudopodia, or
"false feet."
Make sketches at intervals of one or two minutes to show the changes in the form
of the body.
2.Observe the peripheral zone of hyaline protoplasm, the ectoplasm, and
compare this with the inner protoplasm, the endoplasm. Observe in detail the
formation of a pseudopodium. Does the endoplasm extend into the pseudopodium?
Can you explain how the movement is caused ?
3.Find a clear space which appears and disappears at intervals; this is the
contractile vacuole. Determine the length of time between successive contractions.
Are the intervals regular? When the vacuole contracts what becomes of the
contents? Do you know its supposed function?
4.Note the oval or rounded nucleus moving with the flowing endoplasm. What
is its structure?
5.Food materials in process of digestion are readily seen. Of what do they
consist? They are contained in gastric vacuoles. By careful watching, it is often
possible to observe the manner in which food is ingested and the manner in which
the undigested matter is egested.
Make a careful drawing of an Amoeba.
Amoeba; of various kinds represent in many respects the simplest type of
protozoan, and are therefore placed in the first class of these animals, the
Sarcodina. The individuals of this class all possess pseudopodia, but many are
quite unlike those of Amceba. Look over the figures of various Rhizopoda.
If time and material permit, study Amoeba verrucosa, Arcella, and Difflugia, and
compare them with Amoeba proteus. Do you understand how the shells of the last
two genera are made, and of what service they are? Why are not shells good for all
forms?
Drawings of these forms are desirable.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 128 of 10
Laboratory #3 Species diversity and structural features of testate amoebae.
1. Avilable testate amoebae.
2. Dissemination of testate amoebae in nature.
Laboratory #4 Species diversity and the structure of the shells of foraminifera.
1. The structure of the shells of foraminifera.
2. Foraminifera species diversity.
Laboratory #5 Avilable flagellated. Species diversity and structural features of
plant flagellates. Parasitic flagellates. Structure and life cycles.
1. The structure of plant flagellates.
2. Species diversity.
3. Making total preparations flagellates staining.
4. Parasitic flagellates.
5. Structure and features of the life cycle
MASTIGOPHORA.
EUGLENA.
Understand its habitat and with what forms it is usually associated.
1.Observe
the
free-swimming
movements
of
the
organism,
and the englenoid changes in the foim of the body
Make drawings showing the changes in the shape of a single in-di vklual
2.Distinguish
anterior
and
posterior
ends.
Is
there
any
dorsoventral differentiation? Note the motile organ, the flagellum. Where is it
attached?
What
relation
does
it
bear
to
the
gullet? How is it directed during locomotion of the organism
Does it serve any other purpose besides locomotion?
3 The green color of Euglena is due to chlorophyll, and this enables the animal
to live in the clearest water, being nourished like a typical green plant, but minute
particles of food are also taken into the endoplasm through the gullet, and thus
Euglena combines holozoic and halophytic methods of nutrition Consider the
bearing of this on the position of Euglena and its allies in the protozoan scale
4.Note the absence of color near the anterior and posterior ends of the organism.
Near the anterior end alao notice the red pigment spot, or stigma. What is its
probable function?
5.Stain a specimen with iodin and look for the nucleus. It is obscured by the
chlorophyll.
Make a drawing showing all oj the points observed. Look through the stock
cultures for other forms of Mastigophora, such as Traclielomonas, Perancina,
Pliacus, etc. It is desirable to make drawings of the different forms
Laboratory #6 Features of the life cycle and structure Sporozoa.
1. Sporozoa structure.
2. Species diversity and life cycles Sporozoa features.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 129 of 10
Laboratory #7 Species diversity and structural features of ciliates.
1. Available ciliates.
2. Some aspects of the development and the development of species of ciliates.
3. Cooking appliances total preparations ciliates staining.
Laboratory #8 Lower tissue. Beam. Type Sponges. Skeletal structure.
Reproduction.
1. The structure of sponges.
2. Features skeleton.
3. Reproduction.
Phylum Porifera: Sponges
Sponges belong to phylum Porifera (po-rif´-er-a) (L. porus, pore, +fera, bearing).
Sponges bear myriads of tiny pores and canals that constitute a filterfeeding system
adequate for their inactive life habit. They are sessile animals and depend on water
currents carried through their unique canal systems to bring them food and oxygen
and to carry away their body wastes. Their bodies are little more than masses of
cells embedded in a gelatinous matrix and stiffened by a skeleton of minute
spicules of calcium carbonate or silica and collagen. They have no organs or true
tissues, and even their cells show a certain degree of independence. As sessile
animals with only negligible body movement, they have not evolved a nervous
system or sense organs and have only the simplest of contractile elements.
So, although they are multicellular, sponges share few of the characteristics of
other metazoan phyla. They seem to be outside the line of evolution leading from
choanoflagellates to other metazoa. For this reason they are often called Parazoa
(Gr. para, beside or alongside of, + zoon, animal).
Sponges vary in size from a few millimeters to the great loggerhead sponges,
which may reach 2 m or more across. Many sponge species are brightly colored
because of pigments in their dermal cells. Red, yellow, orange, green, and purple
sponges are not uncommon. However, color fades quickly when sponges are
removed from water. Some sponges, including the simplest, are radially
symmetrical, but many are quite irregular in shape. Some stand erect, some are
branched or lobed, and others are low, even encrusting, in form (Figure 12-4).
Some bore holes into shells or rocks.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 130 of 10
Figure 12-4
Some growth habits and forms of sponges.
Most of the 5000 or more sponge species are marine, although some 150 species
live in fresh water. Marine sponges are abundant in all seas and at all depths, and a
few even exist in brackish water. Although their embryos are free swimming,
adults are always attached, usually to rocks, shells, corals, or other submerged
objects. Some bottom-dwelling forms even grow on sand or mud. Their growth
patterns often depend on shape of the substratum, direction and speed of water
currents, and availability of space, so that the same species may differ markedly in
appearance under different environmental conditions. Sponges in calm waters may
grow taller and straighter than those in rapidly moving waters.
Many animals (crabs, nudibranchs, mites, bryozoans, and fish) live as commensals
or parasites in or on sponges. Larger sponges particularly tend to harbor a large
variety of invertebrate commensals. On the other hand, sponges grow on many
other living animals, such as molluscs, barnacles, brachiopods, corals, or hydroids.
Some crabs attach pieces of sponge to their carapace for camouflage and for
protection, since most predators seem to find sponges distasteful. Some reef fishes,
however, graze on shallow-water sponges.
Sponges are an ancient group, with an abundant fossil record extending back to the
early Cambrian period and even, according to some claims, the Precambrian.
Living poriferans traditionally have been assigned to three classes: Calcarea (with
calcareous spicules), Hexactinellida (six-rayed siliceous spicules), and
Demospongiae (with a skeleton of siliceous spicules or spongin [a specialized
collagen] or both). A fourth class (Sclerospongiae) was erected to contain sponges
with a massive calcareous skeleton and siliceous spicules. Some zoologists
maintain that known species of sclerosponges can be placed in the traditional
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 131 of 10
classes of sponges (Calcarea and Demospongiae); thus we do not need a new class.
Characteristics of Phylum Porifera
1. Multicellular; body a loose aggregation of cells of mesenchymal origin>
2. Body with pores (ostia), canals, and chambers that serve for passage of
water
3. Mostly marine; all aquatic
4. Radial symmetry or none
5. Epidermis of flat pinacocytes; most interior surfaces lined with flagellated
collar cells (choanocytes) that create water currents; a gelatinous protein
matrix called mesohyl (mesoglea) contains amebocytes of various types and
skeletal elements
6. Skeletal structure of fibrillar collagen (a protein) and calcareous or siliceous
crystalline spicules, often combined with variously modified collagen
(spongin)
7. No organs or true tissues; digestion intracellular; excretion and respiration
by diffusion
8. Reactions to stimuli apparently local and independent; nervous system
probably absent
9. All adults sessile and attached to substratum
10.Asexual reproduction by buds or gemmules and sexual reproduction by eggs
and sperm; freeswimming ciliated larvae
Form and Function
The only body openings of these unusual animals are pores, usually many tiny
ones called ostia for incoming water, and a few large ones called oscula (sing.,
osculum) for water outlet. These openings are connected by a system of canals,
some of which are lined with peculiar flagellated collar cells called choanocytes,
whose flagella maintain a current of environmental water through the canals.
Water enters the canals through a multitude of tiny incurrent pores (dermal ostia)
and leaves by way of one or more large oscula. Choanocytes not only keep the
water moving but also trap and phagocytize food particles that are carried in the
water. Cells lining the passageways are very loosely organized. Collapse of the
canals is prevented by the skeleton, which, depending on the species, may be
composed of needlelike calcareous or siliceous spicules, a meshwork of organic
spongin fibers, or a combination of the two.
Sessile animals make few movements and therefore need little in the way of
nervous, sensory, or locomotor parts. Sponges apparently have been sessile from
their earliest appearance and have never acquired specialized nervous or sensory
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 132 of 10
structures, and they have only the very simplest of contractile systems.
Types of Canal Systems
Most sponges have one of three types of canal systems: asconoid, syconoid, or
leuconoid (Figure 12-5).
Figure 12-5
Three types of sponge structure. The degree of complexity from simple asconoid to
complex leuconoid type
has involved mainly the water-canal and skeletal systems, accompanied by
outfolding and branching of the
collar-cell layer. The leuconoid type is considered the major plan for sponges, for it
permits greater size and
more efficient water circulation.
Asconoids: Flagellated Spongocoels Asconoid sponges have the simplest
organization. They are small and tube shaped. Water enters through microscopic
dermal pores into a large cavity called a spongocoel, which is lined with
choanocytes. Choanocyte flagella pull water through the pores and expel it through
a single large osculum (see Figure 12-5). Leucosolenia (Gr. leukos, white, + solen,
pipe) is an asconoid type of sponge. Its slender, tubular individuals grow in groups
attached by a common stolon, or stem, to objects in shallow
seawater. Clathrina (L. clathri, lattice work) is an asconoid with bright yellow,
intertwined tubes (Figure 12-6). Asconoids are found only in the Calcarea.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 133 of 10
Figure 12-6
Clathrina canariensis (class Calcarea) is common on Caribbean reefs in caves and
under ledges.
Syconoids: Flagellated Canals Syconoid sponges look somewhat like larger
editions of asconoids, from which they were derived. They have a tubular body and
single osculum, but the body wall, which is thicker and more complex than that of
asconoids, contains choanocyte-lined radial canals that empty into the spongocoel
(see Figure 12-5). The spongocoel in syconoids is lined with epithelial-type cells
rather than flagellated cells as in asconoids. Water enters through a large number
of dermal ostia into incurrent canals and then filters through tiny openings
called prosopylesinto the radial canals (Figure 12-7). There food is ingested by the
choanocytes, whose flagella force the water through internal pores (apo-pyles) into
the spongocoel. From there it emerges through an osculum. Syconoids do not
usually form highly branched colonies as asconoids do. During development,
syconoid sponges pass through an asconoid stage; then flagellated canals form by
evagination of the body wall. Their development provides evidence that syconoid
sponges were derived from asconoid ancestral stock. Syconoids are found in
classes Calcarea and Hexactinellida. Sycon (Gr. sykon, a fig) is a commonly
studied example of the syconoid type of sponge (see Figure 12-5).
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 134 of 10
Figure 12-7
Cross section through wall of sponge Sycon, showing canal system.
Leuconoids: Flagellated Chambers Leuconoid organization is the most complex
of the sponge types and permits an increase in sponge size. Most leuconoids form
large masses with numerous oscula (Figure 12-8). Clusters of flagellated chambers
are filled from incurrent canals and discharge water into excurrent canals that
eventually lead to the osculum (Figure 12-5). Most sponges are of the leuconoid
type, which occurs in most Calcarea and in all other classes.
Figure 12-8
This orange demosponge, Mycale laevis, often grows beneath platelike colonies of
the stony coral,
Montastrea annularis. The large oscula of the sponge are seen at the edges of the
plates. Unlike some other
sponges, Mycale does not burrow into the coral skeleton and may actually protect
coral from invasion by
more destructive species. Pinkish radioles of a Christmas tree worm,
Spirobranchus giganteus (phylum
Annelida, class Polychaeta) also project from the coral colony. An unidentified
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 135 of 10
reddish sponge can be seen to
the right of the Christmas tree worm.
These three types of canal systems— asconoid, syconoid, and leuconoid—
demonstrate an increase in complexity and efficiency of the water pumping
system, but they do not imply an evolutionary or developmental sequence. The
leuconoid grade of construction has evolved independently many times in sponges.
Possession of a leuconoid plan is of clear adaptive value; it increases the
proportion of flagellated surfaces compared with the volume, thus providing more
collar cells to meet food demands.
Figure 12-9
Small section through sponge
wall, showing four types of
sponge cells. Pinacocytes are
protective and contractile;
choanocytes create water
currents and engulf food
particles; archaeocytes have a
variety of functions; collencytes
secrete collagen.
Types of Cells
Sponge cells are loosely arranged in a gelatinous matrix called mesohyl(mesoglea,
mesenchyme) (Figures 12-7 and 12-9). The mesohyl is the “connective tissue” of
sponges; in it are found various ameboid cells, fibrils, and skeletal elements.
Several types of cells occur in sponges.
Pinacocytes The nearest approach to a true tissue in sponges is arrangement of
the pinacocyte cells of the pinacoderm (Figure 12-9). These are thin, flat,
epithelial-type cells that cover the exterior surface and some interior surfaces.
Some are T-shaped, with their cell bodies extending into the mesohyl. Pinacocytes
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 136 of 10
are somewhat contractile and help regulate surface area of a sponge. Some pinacocytes are modified as contractile myocytes, which are usually arranged in
circular bands around oscula or pores, where they help regulate rate of water flow.
Choanocytes Choanocytes, which line flagellated canals and chambers, are ovoid
cells with one end embedded in mesohyl and the other exposed. The exposed end
bears a flagellum surrounded by a collar (Figures 12-9 and 12-10). Electron
microscopy shows that the collar is made up of adjacent microvilli, connected to
each other by delicate microfibrils, forming a fine filtering device for straining
food particles from water (Figure 12-10B and C). The beat of a flagellum pulls
water through the sievelike collar and forces it out through the open top of the
collar. Particles too large to enter the collar become trapped in secreted mucus and
slide down the collar to the base where they are phagocytized by the cell body.
Larger particles have already been screened out by the small size of the dermal
pores and prosopyles. Food engulfed by the cells is passed on to a neighboring
archaeocyte for digestion.
Figure 12-10
Food trapping by sponge cells. A, Cutaway section of canals showing cellular
structure and direction of
water flow. B, Two choanocytes and C, structure of the collar. Small red arrows
indicate movement of food
particles.
Archaeocytes Archaeocytes are ameboid cells that move about in the mesohyl
(Figure 12-9) and carry out a number of functions. They can phagocytize particles
at the pinacoderm and receive particles for digestion from choanocytes.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 137 of 10
Archaeocytes apparently can differentiate into any of the other types of more
specialized cells in the sponge. Some, called sclerocytes, secrete spicules. Others,
called spongocytes, secrete the spongin fibers of the skeleton,
andcollencytes secrete fibrillar collagen. Lophocytes secrete large quantities of
collagen but are distinguishable morphologically from collencytes.
Types of Skeletons
Its skeleton gives support to a sponge, preventing collapse of canals and chambers.
The major structural protein in the animal kingdom is collagen, and fibrils of
collagen are found throughout the intercellular matrix of all sponges. In addition,
various Demospongiae secrete a form of collagen traditionally known as spongin.
Several types of spongin, differing in chemical composition and form (fibers,
spicules, filaments, spongin surrounding spicules, and so on) are found in various
demosponges. Demospongiae also secrete siliceous spicules. Calcareous sponges
secrete spicules composed mostly of crystalline calcium carbonate and have one,
three, or four rays (Figure 12-11). Glass sponges have siliceous spicules with six
rays arranged in three planes at right angles to each other. There are many
variations in the shape of spicules, and these structural variations are of taxonomic
importance.
Figure 12-11
A, Types of spicules found in sponges. An amazing diversity, complexity, and
beauty of form occurs among
the many types of spicules. B, Some sponge body forms.
Sponge Physiology
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 138 of 10
All activities of a sponge depend on the current of water flowing through its body.
A sponge pumps a remarkable amount of water. Leuconia (Gr. leukos, white), for
example, is a small leuconoid sponge about 10 cm tall and 1 cm in diameter. It is
estimated that water enters through some 81,000 incurrent canals at a velocity of
0.1 cm/second. However, because Leuconia has more than 2 million flagellated
chambers whose combined diameter is much greater than that of the canals, water
flow through chambers slows to 0.001 cm/second. Such a flow rate allows ample
opportunity for food capture by collar cells. All water is expelled through a single
osculum at a velocity of 8.5 cm/second: a jet force capable of carrying waste
products some distance away from the sponge. Some large sponges can filter 1500
liters of water a day.
Sponges feed primarily on particles suspended in the water pumped through their
canal systems. Detritus particles, planktonic organisms, and bacteria are consumed
nonselectively in the size range from 50 µm (average diameter of ostia) to 0.1 µm
(width of spaces between microvilli of choanocyte collar). Pinacocytes may
phagocytize particles at the surface, but most larger particles are consumed in the
canals by archaeocytes that move close to the lining of the canals. The smallest
particles, accounting for about 80% of the particulate organic carbon, are
phagocytized by choanocytes. Sponges also absorb dissolved nutrients from the
water passing through the system. Protein molecules are taken into choanocytes by
pinocytosis.
Digestion is entirely intracellular (occurs within cells), and present evidence
indicates that archaeocytes perform this chore. Choanocytes pass particles of food
to archaeocytes for digestion.
There are no respiratory or excretory organs; both functions apparently occur by
diffusion in individual cells. Contractile vacuoles are found in archaeocytes and
choanocytes of freshwater sponges.
The only visible activities and responses in sponges, other than propulsion of
water, are slight alterations in shape and closing and opening of incurrent and
excurrent pores, and these movements are very slow. The most common response
is closure of the oscula. Apparently excitation spreads from cell to cell, although
some zoologists point to the possibility of coordination by means of substances
carried in the water currents, and some zoologists have tried, not very successfully,
to demonstrate presence of nerve cells.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 139 of 10
Figure 12-12
Section through a gemmule of a
freshwater sponge (Spongillidae).
Gemmules are a mechanism for survival
of the harsh conditions of winter. On
return of favorable conditions, the
archaeocytes exit through the micropyle
to form a new sponge. The
archaeocytes of the gemmule give rise
to all the cell types of the new sponge
structure.
Reproduction
Sponges reproduce both asexually and sexually. Asexual reproductionoccurs by
means of bud formation and by regeneration following fragmentation. External
buds, after reaching a certain size, may become detached from the parent and float
away to form new sponges, or they may remain to form colonies. Internal buds,
or gemmules (Figure 12-12), are formed in freshwater sponges and some marine
sponges. Here, archaeocytes collect in the mesohyl and become surrounded by a
tough spongin coat incorporating siliceous spicules. When the parent animal dies,
the gemmules survive and remain dormant, preserving the species during periods
of freezing or severe drought. Later, cells in the gemmules escape through a special
opening, the micropyle, and develop into new sponges. Gemmulation in
freshwater sponges (Spongillidae) is thus an adaptation to changing seasons.
Gemmules are also a means of colonizing new habitats, since they can spread by
streams or animal carriers. What prevents gemmules from hatching during the
season of formation rather than remaining dormant? Some species secrete a
substance that inhibits early germination of gemmules, and gemmules do not
germinate as long as they are held in the body of the parent. Other species undergo
maturation at low temperatures (as in winter) before they germinate. Gemmules in
marine sponges also seem to be an adaptation to pass the cold of winter; they are
the only form in whichHaliclona loosanoffi exists during the colder parts of the
year in the northern part of its range.
In sexual reproduction most sponges are monoecious (have both male and female
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 140 of 10
sex cells in one individual). Sperm arise from transformation of choanocytes. In
Calcarea and at least some Demospongiae, oocytes also develop from choanocytes;
in other demosponges oocytes apparently are derived from archaeocytes. Most
sponges are viviparous; after fertilization the zygote is retained in and derives
nourishment from the parent, and a ciliated larva is released. In such sponges,
sperm are released into the water by one individual and taken into the canal system
of another. There choanocytes phagocytize the sperm, then the choanocytes
transform into carrier cells, which carry the sperm through the mesohyl to oocytes.
Other sponges are oviparous, and both oocytes and sperm are expelled into the
water. The free-swimming larva of most sponges is a solidbodied parenchymula (Figure 12-13A). The outwardly directed, flagellated cells
migrate to the interior after the larva settles and become choanocytes in the
flagellated chambers.
Figure 12-13
A, Development of demosponges. B, Development of the syconoid sponge Sycon.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 141 of 10
Calcarea and a few Demospongiae have a very strange developmental pattern. A
hollow blastula, called anamphiblastula (Figure 12-13B), develops, with
flagellated cells toward the interior. The blastula then turns inside out(inversion),
the flagellated ends of the cells becoming directed to the outside! Flagellated
cells (micromeres) of the larva are at one end, and larger, nonflagellated
cells (macromeres) are at the other. In contrast to other metazoan embryos, the
micromeres invaginate into and are overgrown by the macromeres. The flagellated
micromeres become choanocytes, archeocytes, and collencytes of the new sponge,
and the nonflagellated cells give rise to pinacoderm and sclerocytes.
Regeneration and Somatic Embryogenesis
Sponges have a tremendous ability to repair injuries and to restore lost parts, a
process called regeneration. Regeneration does not imply a reorganization of the
entire animal, but only of the wounded portion.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 142 of 10
On the other hand, if a sponge is cut into small fragments, or if the cells of a
sponge are entirely dissociated and are allowed to fall into small groups, or
aggregates, entire new sponges can develop from these fragments or aggregates of
cells. This process has been termed somatic embryogenesis. Somatic
embryogenesis involves a complete reorganization of the structure and functions of
participating cells or bits of tissue. Isolated from influence of adjoining cells, they
can realize their own potential to change in shape or function as they develop into a
new organism.
A great deal of experimental work has been done in this field. The process of
reorganization appears to differ in sponges of differing complexity. There is still
some controversy concerning just what mechanisms cause adhesion of the cells
and the share that each type of cell plays in the formative process.
Class Calcarea (Calcispongiae)
Calcarea (also called Calcispongiae) are calcareous sponges, so called because
their spicules are composed of calcium carbonate. Spicules are straight (monaxons)
or have three or four rays. These sponges tend to be small—10 cm or less in
height—and tubular or vase shaped. They may be asconoid, syconoid, or leuconoid
in structure. Though many are drab in color, some are bright yellow, red, green, or
lavender. Leucosolenia and Sycon (often called Scypha orGrantia by biological
supply companies) are marine shallowwater forms commonly studied in the
laboratory.Leucosolenia is a small asconoid sponge that grows in branching
colonies, usually arising from a network of horizontal, stolonlike tubes (Figure 126). Sycon is a solitary sponge that may live singly or form clusters by budding. The
vase-shaped, typically syconoid animal is 1 to 3 cm long, with a fringe of straight
spicules around the osculum to discourage small animals from entering.
Class Hexactinellida (Hyalospongiae): Glass Sponges
Glass sponges make up class Hexactinellida (or Hyalospongiae). Nearly all are
deep-sea forms that are collected by dredging. Most are radially symmetrical, with
vase- or funnelshaped bodies usually attached by stalks of root spicules to a
substratum (Figure 12-11, Euplectella) (N. L. from Gr. euplektos, well plaited).
They range from 7.5 cm to more than 1.3 m in length. Their distinguishing features
are a skeleton of six-rayed siliceous spicules that are commonly bound together
into a network forming a glasslike structure and a trabecular net of living tissue
produced by the fusion of pseudopodia of archaeocytes. Within the trabecular net
are elongated, finger-shaped chambers lined with choanocytes and opening into the
spongocoel. The osculum is unusually large and may be covered by a sievelike
plate of silica. There is no pinacoderm or gelatinous mesohyl, and both the external
surface and the spongocoel are lined with a trabecular net. The skeleton is rigid,
and muscular elements (myocytes) appear to be absent. The general arrangement
of the chambers fits glass sponges into both syconoid and leuconoid types. Their
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 143 of 10
structure is adapted to the slow, constant currents of sea bottoms, because channels
and pores of the sponge wall are relatively large and uncomplicated and permit an
easy flow of water. Little, however, is known about their physiology, doubtless
because of their deep-water habitat.
The latticelike network of spicules found in many glass sponges is of exquisite
beauty, such as that of Euplectella, or Venus’ flower basket (Figure 12-11), a
classic example of Hexactinellida.
Class Demospongiae
Class Demospongiae contains 95% of living sponge species, including most larger
sponges. Spicules are siliceous but are not six rayed, and they may be bound
together by spongin or may be absent altogether. All members of the class are
leuconoid, and all are marine except one family, the Spongillidae, or freshwater
sponges.
Freshwater sponges are widely distributed in well-oxygenated ponds and streams,
where they encrust plant stems and old pieces of submerged wood. They may
resemble a bit of wrinkled scum, be pitted with pores, and be brownish or greenish
in color. Common genera are Spongilla (L. spongia, from Gr. spongos, sponge)
and Myenia. Freshwater sponges are most common in midsummer, although some
are more easily found in the fall. They die and disintegrate in late autumn, leaving
gemmules to produce the next year’s population. They also reproduce sexually.
Marine Demospongiae are quite varied and may be quite striking in color and
shape (Figure 12-14). Some are encrusting; some are tall and fingerlike; some are
low and spreading; some bore into shells; and some are shaped like fans, vases,
cushions, or balls (Figure 12-14). Loggerhead sponges may grow several meters in
diameter.
Figure 12-14
Marine Demospongiae on Caribbean coral reefs. A, Pseudoceratina crassa is a colorful
sponge growing at
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 144 of 10
moderate depths. B, Ectyoplasia ferox is irregular in shape and its oscula form small,
volcano-like cones. It is toxic
and may cause skin irritation if touched. C, Monanchora unguifera with commensal brittle
star, Ophiothrix suensoni
(phylum Echinodermata, class Ophiuroidea).
So-called bath sponges (Spongia, Hippospongia) belong to the group called horny
sponges, which have spongin skeletons and lack siliceous spicules entirely.
Phylogeny and Adaptive Radiation
Phylogeny
Sponges originated before the Cambrian period. Two groups of calcareous
spongelike organisms occupied early Paleozoic reefs. The Devonian period saw
rapid development of many glass sponges. The possibility that sponges arose from
choanoflagellates (protozoa that bear collars and flagella) earned support for a
time. However, many zoologists opposed that hypothesis because sponges do not
acquire collars until later in their embryological development. The outer cells of
the larvae are flagellated but not collared, and they do not become collar cells until
they become internal. Also, collar cells are found in certain corals and
echinoderms, so they are not unique to the sponges.
However, these objections are countered by evidence based on the sequences of
ribosomal RNA. This evidence supports the hypothesis that choanoflagellates and
metazoans are sister groups. It suggests also that sponges and Eumetazoa are sister
groups, with Porifera having separated before the origin of radiates and
placozoans, but sharing a common ancestor.
Adaptive Radiation
Porifera are a highly successful group that includes several thousand species and a
variety of marine and freshwater habitats. Their diversification centers largely on
their unique water-current system and its various degrees of complexity.
Proliferation of flagellated chambers in leuconoid sponges was more favorable to
an increase in body size than that of asconoid and syconoid sponges because
facilities for feeding and gaseous exchange were greatly enlarged.
Classification of Phylum Porifera
Class Calcarea (cal-ca´re-a) (L. calcis, lime) (Calcispongiae). Have spicules of
calcium carbonate that often form a fringe around the osculum (main water outlet);
spicules needle shaped or three or four rayed; all three types of canal systems
(asconoid, syconoid, leuconoid) represented; all marine. Examples:
Class Hexactinellida (hex-ak-tin-el´i-da) (Gr. hex, six, + aktis, ray, + L. -
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 145 of 10
ellus, dim. suffix) (Hyalospongiae). Have six-rayed, siliceous spicules extending
at right angles from a central point; spicules often united to form network; body
often cylindrical or funnel shaped; flagellated chambers in simple syconoid or
leuconoid arrangement; habitat mostly deep water; all marine. Examples: Venus’
flower basket (Euplectella), Hyalonema.
Class Demospongiae (de-mo-spun´jee) (Gr. demos, people, + spongos, sponge).
Have siliceous spicules that are not six rayed, or spongin, or both; leuconoid-type
canal systems; one family found in fresh water; all others marine.
Examples: Thenea, Cliona, Spongilla, Myenia, and all bath sponges.
Laboratory #9 -10 Species diversity and structural features of the type
Coelenterates.
1. Structure of various kinds of cnidarians.
2. Species diversity of cnidarians.
COELENTERATA.
WITH A SINGLE CONTINUOUS COELENTERON OR GASTRO-VASCULAR CAVITY. WITH
THE EXCEPTION OF THE CTENOPHORA ALL HAVE NETTLE CELLS.
THERE ARE TWO CELLULAR LAYERS AND A MESOGLEA.
Class 1. HYDROZOA.
COELENTERON SIMPLE, WITHOUT SEPTA. GONADS USUALLY ECTODERMAL.
FULLY FORMED MEDUSAE HAVE A VELUM.
Class 2. SCYPHOZOA.
BODY-WALL OF POLYP THROWN INTO FOUR RIDGES WHICH PROJECT INTO THE
COELENTERON. MEDUSAE WITHOUT VELUM AND WITH GASTRIC TENTACLES.
MEDUSOID FORM PREDOMINATING.
Class 3. ANTHOZOA.
WITH A STOMODAEUM, AND WITH MESENTERIES EXTENDING INTO THE
COELENTERON. FIXED FORMS.
HYDRA,
HYDROZOA.
HYDRA. (Fresh-water Polyp.)
COMMON FRESH-WATER COELENTERATE,
THE ONLY
IS FREQUENTLY
FOUND IN JARS OF WATER TAKEN FROM QUIET POOLS OR SLUGGISH STREAMS THAT
CONTAIN LILY-PADS, DECAYING LEAVES, AND OTHER VEGETABLE MATTER. THE
ANIMALS MAY FREQUENTLY BE FOUND BY EXAMINING THE SURFACES OF SUBMERGED
LEAVES, BUT IT IS USUALLY BETTER TO ALLOW SUCH MATERIAL TO STAND IN GLASS
JARS FOR A DAY OR TWO, AS THE ANIMALS THEN TEND TO COLLECT ON THE LIGHTER
SIDES OF THE VESSELS. THEY ARE EASILY KEPT IN BALANCED AQUARIA.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 146 of 10
EXAMINE
SPECIMENS IN AN AQUARIUM AND FIND WHAT YOU CAN ABOUT THEIR
MODE OF LIFE. DO THEY FORM COLONIES?
PLACE A SPECIMEN IN A WATCH-GLASS OF WATER AND EXAMINE IT WITH A LENS
1 WHAT IS ITS SHAPE AND COLOR? IS IT ATTACHED? IF SO, BY WHAT PART OF
THE BODY? NOTICE THE CIRCLET OF tentacles. HOW MANY ARE THERE? COMPARE
NOTES WITH OTHERS AND SEE IF ALL HAVE THE SAME NUMBER HOW ARE THEY
PLACED?
2. DOES THE HYDRA MOVE ITS BODY OR TENTACLES? IS IT SENSITIVE? HOW DO
YOU KNOW?
3. EXAMINE WITH A LOW POWER OF THE MICROSCOPE AND REVIEW THE ABOVE
POINTS. YOU MAY ALSO BE ABLE TO SEE THE mouth AROUND WHICH THE TENTACLES
ARE ARRANGED.
Make two drawings, one showing the animal expanded and the other contracted.
PLACE YOUR SPECIMEN ON a SLIDE UNDER A COVER-GLASS THAT IS SUPPORTED BY
THE EDGE OF ANOTHER COVER-GLASS, SO IT CAN BE EXAMINED WITH a HIGH POWER.
Be careful not to crush it. NOTICE:
4.THE OUTER LAYER, ectoderm. WHAT IS ITS COLOR? IS IT CONTINUOUS OVER THE
WHOLE OUTER SURFACE? DOES IT VARY IN THICKNESS? ARE THE CELLS OF WHICH IT
IS COMPOSED APPARENTLY ALL ALIKE?
5.THE INNER LAYER, endoderm. WHAT IS ITS COLOR? IF COLOR IS PRESENT, IS IT
EVENLY DIFFUSED OR IS IT COLLECTED IN SPECIAL BODIES? ARE THE CELLS OF WHICH
THE ENDODERM IS COMPOSED APPARENTLY ALL ALIKE? DO THEY DIFFER IN
APPEARANCES FROM THOSE OF THE ECTODERM OTHER THAN IN COLOR? IF THE
SPECIMEN IS NOT DEEPLY COLORED, LOOK FOR FLAGELLA MOVING IN THE INTERNAL
CAVITY.
6. EXAMINE THE ECTODERM OF THE TENTACLES CAREFULLY AND NOTICE THAT
EACH OF THE LARGE, ROUNDED, CLEAR CELLS, THE nematocysts, SHOWS A RATHER
INDEFINITE STREAK RUNNING FROM ITS OUTER END, BACK INTO THE INTERIOR. SEE IF
YOU CAN FIND THE TRIGGER (cnidocil) ON ANY OF THESE CELLS.
Draw a portion of a tentacle showing the distribution of the nematocysts.
7.PLACE YOUR SPECIMEN UNDER THE LOW POWER OF THE MICROSCOPE, CAREFULLY RUN IN a DROP OF SAFFRANIN, AND SEE IF ANY OF THE
NEMATOCYSTS ARE DISCHARGED WHEN THE SAFFRANIN TOUCHES THEM.
EXAMINE WITH A HIGH POWER AND NOTICE THE APPEARANCE OF THE
THREAD. NOTICE THE CHANGE IN THE SHAPE OF THE NEMATOCYSTS
THAT HAVE DISCHARGED. SEE IF YOU CAN FIND TWO KINDS.
Make an enlarged drawing of an exploded nematocyst.
8.EXAMINE PREPARED TRANSVERSE SECTIONS OF HYDRA. NOTICE
THAT THE BODY IS COMPOSED OF TWO LAYERS OF CELLS, BETWEEN WHICH
IS AN ALMOST STRUCTURELESS THIN LAYER DO THE CELLS OF THE TWO
LAYERS DIFFER IN SIZE, SHAPE, AND STRUCTURE? DO YOU FIND MORE
THAN ONE KIND OF CELL IN EACH OR EITHER OF THESE LAYERS? WHERE
ARE THEY? WHAT ARE THEY?
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 147 of 10
Make a careful drawing of the section showing the arrangement as you see it.
EXAMINE LONGITUDINAL SECTIONS, FOR DIFFERENCES IN THE CHARACTER OF THE
ECTODERM AND ENDODERM IN DIFFERENT PARTS OF THE BODY.
9 Reproduction EXAMINE LIVING SPECIMENS IN A WATCH-GLASS OF WATER FOR
BUD FORMATION AND FOR SEXUAL ORGANS Spermarics ARE JUST BENEATH THE
TENTACLES, ovaries, LOWER DOWN; buds MAY BE FOUND AT DIFFERENT LEVELS.
WHAT LAYERS OF CELLS IS INVOLVED IN THE FORMATION OF EACH OF THESE?
EGGS ARE NOT FORMED AT ALL SEASONS OF THE YEAR AND VARY GREATLY IN
APPEARANCE ACCORDING TO THEIR STAGE OF DEVELOPMENT.
Make drawings of the stages of reproduction that you find.
SCYPHOZOA.
AURELIA.
THIS FORM IS ONE OF THE COMMON JELLY-FISHES, AND IS FOUND FLOATING FREELY
IN THE WATER. IT IS FREQUENTLY WASHED UP ON SHORE. TO BE APPRECIATED THESE
MEDUSAS SHOULD BE SEEN AS THEY OCCUR AT THE SURFACE OF THE SEA, BEFORE
THEY HAVE BEEN HANDLED OR INJURED. FREQUENTLY VAST NUMBERS MAY BE SEEN
TOGETHER, ALL GENTLY PULSATING AND THUS KEEPING NEAR THE SURFACE. THE
MOVEMENT IS VERY DIFFERENT FROM THAT OF MOST HYDROZOAN MEDUSA, BEING
VERY DELIBERATE AND GRACEFUL
IF LIVING MATERIAL IS OFFERED, STUDY THE METHOD OF LOCOMOTION AND
COMPARE IT WITH THE LOCOMOTION OF Gonioncurus. LIKE THE LATTER, THE
DISCOID ANIMAL PRESENTS ex-umbrellar (ABORAL) AND sub-umbrellar (ORAL)
SURFACES, BUT THE EDGES OF THE DISK ARE INDENTED, FRINGED WITH VERY
NUMEROUS SHORT TENTACLES, AND A VELUM IS WANTING. WHAT DIFFERENCE DOES
THE VELUM MAKE IN LOCOMOTION ?
THE EX-UMBRELLAR SURFACE PRESENTS LITTLE OF INTEREST. IN THE LIVE
SPECIMENS, HOWEVER, PROVE THAT THE ANIMAL IS SENSITIVE OVER THIS AREA AS
ELSEWHERE.
PRESERVED AND HARDENED MATERIAL IS BETTER THAN LIVING FOR THE STUDY OF
THE REST OF THE ANATOMY OF THIS FORM. WITH A SPECIMEN IN WATER IN A FINGERBOWL, WITH A BLACK TILE FOR THE BACKGROUND, FIND THE FOLLOWING FROM THE
SUB-UMBRELLAR SURFACE:
1. THE SHAPE OF THE ANIMAL. IS THE MARGIN PERFECTLY CIRCULAR OR
REGULARLY INDENTED? ARE ALL OF THE MARGINAL PORTIONS SIMILAR?
2. FOUR LARGE, FRINGED oral arms OR lips HANG FROM THE CORNERS OF THE
NEARLY SQUARE mouth, WHICH IS LOCATED IN THE CENTER. NOTICE HOW EACH
ARM IS SIMILAR TO A LONG, NARROW LEAF, WITH THE SIDES FOLDED ESPECIALLY
ALONG THEIR MARGINS. EXAMINE THE ARMS FOR NEMATOCYSTS. DO YOU
UNDERSTAND HOW THE ANIMAL GETS ITS FOOD? IF THE ARM EDGES APPEAR TO
BE COVERED WITH DARK SPECKS AND GRANULES, EXAMINE TO SEE IF embryos
MAY NOT BE ENTANGLED.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 148 of 10
3 THE MOUTH IS FOUND TO LEAD BY A SHORT gullet INTO A RATHER SPACIOUS
stomach, WHICH IS PRODUCED IN THE REGION BETWEEN EACH TWO CORNERS OF THE
MOUTH TO FORM A gastric pouch. DETERMINE THE SHAPE OF THE STOMACH.
4. THE REMAINING PARTS OF THE DIGESTIVE (AND ALSO IN THIS CASE
CIRCULATORY) SYSTEM INCLUDE THE NUMEROUS radial canals AND THE SINGLE
circumferential canal.
(a) DIRECTLY BENEATH EACH ORAL ARM A per-radial canal IS GIVEN OFF, WHICH,
AT A SHORT DISTANCE FROM THE STOMACH, GIVES OFF A BRANCH ON EITHER
SIDE. THE PER-RADIAL CANAL PROPER USUALLY CONTINUES STRAIGHT TO THE
MARGINAL CIRCUMFERENTIAL CANAL, WITHOUT FURTHER SUBDIVISION, BUT
THE TWO SIDE BRANCHES ABOVE MENTIONED IN TURN SUBDIVIDE SEVERAL
TIMES.
FROM THE PERIPHERAL WALL OF EACH GASTRIC POUCH THREE CANALS PASS
TOWARD THE MARGIN; THE MIDDLE ONE (inter-radial canal) BRANCHES SOMEWHAT
AFTER THE MANNER OF THE PER-RADIAL CANALS, BUT THE OTHER TWO (ad-radial
canals) CONTINUE TO THE CIRCULAR CANAL WITHOUT FURTHER BRANCHING
5. THE POSITION OF THE GASTRIC POUCHES IS MADE CLEARLY MANIFEST BY THE
gonads, WHICH LIE ON THE FLOOR OF THE POUCHES, AS FRILL LIKE STRUCTURES,
HORSESHOE-SHAPED, WITH THEIR OPEN SIDES TOWARD THE MOUTH THE ova OR
spermatozoa ARE SHED INTO THE STOMACH AND PASS OUT OF THE MOUTH EMBRYOS
IN VARIOUS STAGES OF DEVELOPMENT MAY FREQUENTLY BE FOUND ADHERING TO
THE ORAL ARMS THE SEXES ARC SEPARATE. ON THE SUB-UMBRELLAR SURFACE,
OPPOSITE EACH GONAD, IS A LITTLE POCKET, THE sub-genital pit, WHICH OPENS
FREELY TO THE OUTSIDE. WHATEVER PURPOSE THIS MAY SERVE, IT DOES NOT
FUNCTION TO CONDUCT THE GENITAL PRODUCTS TO THE OUTSIDE.
6. PARALLEL WITH THE INNER OR CONCAVE BORDER OF EACH GONAD IS A ROW OF
DELICATE gastric filaments. THESE ARE SUPPLIED WITH STINGING CELLS, AND THEY
MAY AID IN KILLING LIVE FOOD TAKEN INTO THE STOMACH THESE STRUCTURES ARE
NOT PRESENT IN THE HYDROZOAN MEDUSA.
7 AT THE MARGINAL EXTREMITY OF EACH PER-RADIAL AND INTER-RADIAL CANAL
THERE IS AN INCISION ON THE EDGE OF THE ANIMAL, IN WHICH THERE ARE SENSORY
ORGANS. IN EACH INCISION FIND:
(a) A tentaculocyst IN THE FORM OF A SHORT, CLUB-LIKE STRUCTURE
CONTAINING A PROLONGATION OF THE CIRCULAR CANAL. AT ITS OUTER
EXTREMITY ARE CALCAREOUS CONCRETIONS OR lithiles, AND A PIGMENT-SPOT
OR ocellus. EACH TENTACULOCYST IS PROTECTED ABORALLY BY A HOOD-LIKE
PROJECTION, AND ON THE SIDES BY MARGINAL LAPPETS.
(b) TWO DEPRESSIONS, ONE ABOVE AND THE OTHER BELOW THE TENTACULOCYST.
THESE HAVE BEEN ASSIGNED OLFACTORY FUNCTIONS, AND ARE CALLED THE
olfactory pits
Make a drawing showing the profile of the entire animal, and show the structure
of at least one quadrant, as seen from the oral surface.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 149 of 10
IF
TIME PERMITS STUDY A DEVELOPMENTAL STAGE, "ephyra," AND COMPARE IT
WITH THE ADULT.
BY WAY OF COMPARISON, EXAMINE DEMONSTRATIONS OF Cyanea, Dactylometra,
Lucernarm, OR OTHER FORMS BELONGING TO THIS GROUP.
ACTINOZOA.
METRIDIUM. (Sea-Anemons.)
SPECIMENS ARE QUITE COMMON ON PILES, AS WELL AS ON ROCKY BOTTOMS, AND
MAY BE EASILY OBSERVED BY MEANS OF A GLASS-BOTTOMED PAIL. MOST OF THE
OBSERVATIONS CAN BE MADE MUCH BETTER ON SPECIMENS IN AQUARIA, BUT IT IS
DESIRABLE TO SEE THEIR NATURAL SURROUNDINGS.
1.NOTICE THE SHAPE AND ATTACHMENT OF EXPANDED, LIVING SPECIMENS IN AN AQUARIUM, OR IN A DEEP FINGER-BOWL THE FREE END,
CALLED THE disk OR peristome, IS FRINGED WITH tentacles, AND THE
ELONGATED mouth IS LOCATED IN THE MIDDLE OF THIS AREA AT ONE
OR BOTH ANGLES OF THE MOUTH THE LIPS ARE THICKENED INTO WHAT IS
CALLED A siphonoglyph.
Make a drawing of the animal.
3. FEED A SPECIMEN WITH
BITS OF MASHED CLAM TO ASCERTAIN
ITS MANNER OF TAKING IN FOOD. DROP BITS ON THE TENTACLES AT ONE
TIME, AND DISK AT ANOTHER.
ENDEAVOR ALSO TO DETERMINE WHETHER THERE ARE CURRENTS CONSTANTLY
PASSING IN OR OUT OF THE MOUTH THAT ARE DUE TO CILIARY ACTION
3 IRRITATE THE ANIMAL AND OBSERVE ITS MANNER OF CONTRACTION. WHEN
FULLY CONTRACTED, IF THE IRRITATION IS CONTINUED, THREADLIKE STRUCTURES,
acontia, ARE THRUST OUT THROUGH MINUTE PORES, cinclidcs, IN THE BODY-WALL.
Make a drawing of the contracted animal
Internal Anatomy— USING PRESERVED MATERIAL, PLACE THE EDGE OF A RAZOR
ACROSS THE PERISTOMIAL AREA, AT RIGHT ANGLES TO THE MOUTH-SLIT, AND DIVIDE
THE ANIMAL FROM DISK TO BASE INTO HALVES.
1. NOTE THE EXTENT OF THE esophagus AND siphonoglyphcs; THEY LEAD INTO THE
calenteric chamber. FIND THE EXTENT OF THIS CHAMBER, AND THE METHOD OF ITS
SUBDIVISION BY DELICATE PARTITIONS, THE mesenteries, OR septa ARE ALL OF THE
MESENTERIES ALIKE?
2. FORMING THE FREE EDGES OF THE MESENTERIES, BELOW THE ESOPHAGUS, ARC THE
CONVOLUTED mesenteric filaments, WHICH ARE SECRETORY ORGANS THAT ARE
PROBABLY EQUIVALENT TO THE GASTRIC FILAMENTS OF THE SCYPHOZOA.
3.QUITE NEAR THE BASES OF THE MESENTERIES ARE THE ATTACHMENTS OF THE
acontia. WHAT RELATION HAVE THEY TO THE MESENTERIC FILAMENTS?
4. ALSO LOCATED ON THE MESENTERIES, AND ARRANGED PARALLEL TO THE
FILAMENTS, BUT BACK FROM THE EDGE A BIT, ARE THE reproductive organs OR
gonads. ARE THEY FOUND ON ALL OF THE MESENTERIES' THE OVA OR SPERMATOZOA
ARE SHED INTO THE COELENTERIC CHAMBER AND PASS OUT THROUGH THE MOUTH
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 150 of 10
CUT
ONE OF THE HALVES OF YOUR SPECIMEN TRANSVERSELY IN THE REGION OF
THE ESOPHAGUS, AND STUDY THE ARRANGEMENTS OF THE MESENTERIES, THEIR
ATTACHMENTS, ETC.
5. HOW MANY PAIRS OF primary mesenteries, I. E , THOSE ATTACHED BOTH TO
THE OUTER BODY-WALL AND TO THE ESOPHAGUS, ARE THERE? THE directive
septa ARE THOSE AT THE ANGLES OF THE ESOPHAGEAL TUBE. THE PORTION OF
THE COELENTERIC CAVITY BETWEEN ANY TWO PAIRS OF MESENTERIES IS
TERMED AN inter-radial chamber. THE SPACE BETWEEN THE TWO
MESENTERIES OF EACH PAIR IS CALLED AN intra-radial chamber.
6. CAREFULLY
DETERMINE THE DISPOSITION OF THE longitudinal retractor
MUSCLES ON THE MESENTERIES. DO THEY OCCUPY SIMILAR POSITIONS ON ALL
OF THE MESENTERIES?
7. EXAMINE THE UPPER PARTS OF THE MESENTERIES FOR OPENINGS, septal
stomata, THAT PUT THE CHAMBERS IN COMMUNICATION
8.ARE THE TENTACLES SOLID OR HOLLOW?
Make a drawing of a longitudinal section and another of a cross-section Put
into these all of the points of the anatomy you have seen.
Laboratory #11 Musculoskeletal system of flat, round and annelids.
1. Features motion worms.
2. Musculoskeletal system worms.
Laboratory #12 System metabolism. The structure of the digestive system of flat,
round and annelids.
1. System metabolism in flat, round and annelids.
2. The structure of the digestive system of flat, round and annelids.
Laboratory #1. System of metabolism. The structure of the digestive system of flat,
round and annelids.
1. System metabolism in flat, round and annelids.
2. The structure of the digestive system of flat, round and annelidsLab 4 –
topic, , methodical recommendations, questions for self-protection and
laboratory classes
Comparison of Parasitic and Free-Living Worms
Objectives:
■ Understand the taxonomic relationships and major features of the worm phyla,
Platyhelminthes, Nematoda and Annelida
■ Learn the external and internal anatomy of Dugesia, Clonorchis, and Ascaris and
become familiar with the external features of the other specimens
■ Learn the defining characteristics of both ectoparasites and endoparasites,
focusing on the structural differences between parasites and free-living forms
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 151 of 10
Introduction:
During this week of our animal diversity survey, we will study three worm
phyla. All of the phyla of worms that we will examine - the annelids, the
nematodes, and the platyhelminthes - contain species that are parasites of humans
(not to mention other animals and plants). You may already be familiar with some
of these creatures: you are likely to encounter leeches (an annelid) simply from
wading in a steam or pond, and if you ever had a dog or cat, you probably took it
to the vet at least once to be treated for worms (such as roundworms and
whipworms, both nematodes, and tapeworms, a platyhelminth).
The parasitic worms that you will examine are for the most part eating and
reproducing machines. Consequently, when studying the parasitic worms, take a
good look at their digestive and reproductive systems, and then compare them to
the digestive and reproductive systems of free-living worms (e.g., earthworms).
1) Phylum Platyhelminthes
The phylum Platyhelminthes (platy, flat; helminth, worm) includes a
diversity of marine, freshwater, and terrestrial worms, plus two rather important
parasitic groups: the flukes and the tapeworms. Like cnidarians (= hydras, jellyfish,
and corals), flatworms have a rather simple body plan and share some features with
them. They also have a few morphological advancements over cnidarians. Some
characteristics of flatworms are:
1) They are triploblastic, as all three primary germ layers (e.g., ectoderm,
endoderm and a middle tissue layer, the mesoderm) form during embryonic
development. As a result, flatworms have well-developed, mesodermal-derived
muscle layers. However, they are acoelomate, lacking a true body cavity.
2) Flatworms lack organs for transporting oxygen to body tissues. As a
consequence, each of their cells must be near the body surface for gas exchange to
take place, resulting in a flattened body plan.
3) Flatworms are bilaterally symmetrical.
4) The digestive system of flatworms, if present, consists of a single opening that
serves as both the mouth and anus. This opening, the mouth, leads into a branched
gastrovascular cavity. Both digestion and absorption of nutrients occur in the
gastrovascular cavity, obviating the need for a well-developed circulatory system.
The phylum is divided into four classes:
Class Turbellaria, free-living marine, freshwater, and terrestrial flatworms. Class
Trematoda, parasitic internal flukes
Class Cestoda, parasitic tapeworms
Class Monogenea, parasitic external flukes
Specimens of Platyhelminthes
We will examine live speciemens (Dugesia) and microscope slides (Dugesia,
Clonorchis, Taenia) representative of free-living and parasitic platyhelminthes.
A) Dugesia, Class Turbellaria, live specimen.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 152 of 10
Obtain a live Dugesia flatworm by sucking it up from the side or bottom of a
glass jar using a medicine dropper. Place the specimen in a small Petri dish,
making sure that it is completely covered by pond water, and examine it under
your dissecting scope.
Dugesia is a common turbellarian (= planarian) that resides in freshwater
steams and ponds. Note your animal's shape, pigmentation, and mode of
locomotion. Dugesia, as well as most free-living flatworms, move over surfaces by
means of cilia on their ventral surface. Note the pigmented eye spots, or ocelli,
located on the triangular "head" of the animal. These eye spots are sensitive only to
light and dark, and are unable to resolve images. On either side of the eye spots are
lateral lobes which serve as chemosensory organs. Cover your culture dish (top
and sides) with a piece of aluminum foil, and place the dish on a dark background
with a microscope light shining on it. After 5 to 10 minutes, remove the foil and
observe where your animal is relative to the light. Is your animal positively or
negatively attracted to light (= phototactic)? How might this behavior be adaptive
for the animal in its natural environment?
Dugesia feeds by extruding its pharynx from a ventrally-located pharyngeal cavity.
The mouth of the pharynx opens into the gastrovascular cavity, which has many
branches (diverticula) to facilitate digestion. Place a small piece of food into the
culture dish and observe the response of your specimen. If you are lucky, you may
be able to see Dugesia extrude its pharynx and suck up food particles like a mini
vacuum cleaner (Figure 1).
Figure 1: Planarian flatworm, Dugesia, feeding (from Pechenik 1991, Biology of
the Invertebrates).
152
B) Dugesia, microscope slide (Figure 2).
Observe a prepared whole mount of Dugesia under low power of your
compound microscope. You should see the eye spots and the diverticula of the
gut. Also look for the "brain", nerve cord, and excretory system.
Figure 2: Dugesia, whole mount (from Stamps, Phillips & Crowe. The laboratory:
a place to do science, 3rd ed.)
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 153 of 10
C) Clonorchis, Class Trematoda, preserved specimen and microscope slide
(Figure 3).
Clonorchis sinensis, the human liver fluke, is a parasitic trematode found in
the bile ducts of humans. Like most parasitic worms, the life cycle of C. sinensis is
extremely complex and involves several hosts. The adult worm sheds eggs into the
bile ducts of its human host, which eventually reach the small intestine and are
passed with feces. If the eggs are ingested by the proper species of aquatic snail,
they hatch into larvae that then progress through a series of asexual stages,
culminating in an infective larval stage known as cercariae. The cercariae are
ciliated, and have a tail for swimming. They pass out of the snail, and then briefly
swim about in the water until they encounter a fish. Then the cercariae penetrate
the muscles of the fish, lose their tails, and remain encysted until the fish is eaten
by the definitive (= final host). These encysted larvae are freed in the human small
intestine after consumption of improperly prepared fish. The immature flukes
migrate through the bile duct and its tributaries throughout the liver, where they
develop into adult worms. If untreated, an infection by Clonorchis can lead to
enlargement and cirrhosis of the liver.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 154 of 10
Figure 3: Photograph of Clonorchis sinensis, with major features identified (from
Pechenik 1991, Biology of the Invertebrates).
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 155 of 10
Figure 4: Schematic of the trematode, Clonorchis (from Hopkins & Smith, 1997,
Introduction to Zoology).
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 156 of 10
Observe a prepared whole mount of Clonorchis under low power of your
compound microscope. Unlike flatworms, flukes have a protective cuticle covering
their bodies (why?). Note the anterior oral sucker around its mouth, for attachment
to host tissues. A muscular pharynx and esophagus lead to a two-branched
intestine (= gastrovascular cavity). Slightly posterior to the branching point of the
intestine is the ventral sucker, or acetabulum, that also serves to attach the
organism to its host's tissues. A small excretory pore is located at the posterior end.
The remaining conspicuous organs are reproductive structures. The large, branched
organs located in the posterior of the organism are the two testes. A vas deferens
connects each testis to a single, median seminal vesicle (not easily seen) that stores
sperm and transports it to a genital pore located anterior to the acetabulum.
The mid-section of the fluke contains the female reproductive structures. An
enormous uterus occupies much of the central region of the worm, and stores eggs.
On either side lateral to the uterus are yolk glands that secrete yolk for egg
formation via yolk ducts (not visible). A small, lobed ovary can be seen posterior
to the uterus, and behind that is a sac-like seminal receptacle for storing sperm
received during copulation.
D) Taenia, Class Cestoda, preserved specimen and microscope slide (Figure
5).
Observe a prepared slide of Taenia under low power of your compound
microscope. Your specimen, Taenia pisiformis, is a tapeworm of carnivores
(notably, dogs), and closely resembles T. solium and T. saginata, common
parasites of humans contracted by eating poorly prepared beef or pork,
respectively. Tapeworms share many features with flukes, including an outer
cuticle, attachment structures, expansive reproductive organs, and complex life
cycles involving intermediate hosts. Unlike flukes, however, tapeworms lack a
mouth and gastrovascular cavity, a consequence of their life in vertebrate organs of
high nutritional activity (i.e., the small intestine). Bathed by food in their host's
intestine, they absorb predigested nutrients across their body surface via diffusion
and possibly, active transport.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 157 of 10
The body of a tapeworm is divided into four main regions. A small scolex
("head") bears suckers and an elevated rostellum with curved hooks; the suckers
are used for attachment to the host's organs. Immediately posterior to the scolex is
a "neck" that produces many proglottids ("segments") by asexual budding. Each
proglottid is potentially a complete reproductive unit containing by male and
female reproductive organs (i.e., each is hermaphroditic). Why might
hermaphroditism be especially advantageous for an internal parasite?
The second region consists of small, immature proglottids nearest to the
neck and scolex.
The third region, or mid-section, consists of mature proglottids, each with
well-developed male and female reproductive organs. These proglottids engage in
internal, cross fertilization. In a mature proglottid, locate the lateral genital pore
that contains both a thin, tubular vagina and a stouter vas deferens. Trace the
vagina posteriorly and note that it passes between two ovaries and terminates at a
shell gland anterior to a yolk gland. Eggs are fertilized and "yolked" before passing
anteriorly into a sac-like uterus. The male reproductive system consists of
numerous small, round testes, each with a tiny tubule that connects to a single vas
deferens, which transports sperm to the genital pore.
The fourth and posterior region of the tapeworm consists of gravid (= "pregnant")
proglottids. In gravid proglottids, most of the gonads are atrophied, leaving only an
enlarged uterus packed with eggs. These gravid proglottids eventually break off
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 158 of 10
from the body of the adult worm, and pass out of the digestive tract in the host's
feces. When a small mammal, such as a rabbit, ingests a proglottid or eggs, the
eggs hatch into larvae that then bore through the intestinal wall and then move
through the circulatory system where they eventually become encysted in muscle
tissue. When the rabbit is eaten by a dog, the encysted larvae are released, and
develop into adult worms. As can be seen from the specimen on display,
tapeworms can be quite large: T. solium, a parasite of the human intestine, can
reach a length of 10 feet!
2) Phylum Nematoda
Nematodes are probably the most abundant and ubiquitous animals on earth,
having invaded virtually every habitat. Most of the approximately 10,000 species
of nematodes are free-living, but many are parasites of animals, including humans.
Trichinella spiralis, for example, is contracted by eating insufficiently cooked pork.
The adult worms develop in the human intestine, releasing larvae which move
through the lymphatic system, eventually ending up in muscle tissues where they
encyst. Other nasty nematode parasites of humans include Necator americanus
(hookworms) and Wuchereria, which results in elephantiasis. Nematodes also are
parasites of plants and can cause enormous crop damage; as a result, some large
universities have departments of plant pathology devoted to the study of plant
pathogenic nematodes.
Noteworthy characteristics of nematodes are:
1) they are triploblastic.
2) they have a pseudocoelom, a cavity incompletely lined by mesodermallyderived tissue.
3) the fluid-filled pseudocoelom functions as a hydrostatic skeleton.
4) they have a complete, one-way digestive tract, having both a mouth and an anus.
5) they have a non-living, protective cuticle covering their bodies.
Specimens of Nematodes
We will examine preserved specimens of Ascaris lumbricoides, commonly
known as the roundworm, an intestinal parasite of humans. Humans contract
Ascaris by ingesting eggs from the soil. Once ingested, the eggs hatch, releasing
larvae. The larvae bore through the small intestine and migrate via the venous and
lymphatic systems to the lungs. There the larvae continue to grow, and pass
through several larval stages. After a few weeks, the larvae are coughed-up,
literally, and then swallowed, where they develop into mature adults in the small
intestine.
A. Ascaris, external morphology (Figure 6)
Examine preserved specimens of male and female ascarids. The male is
smaller, and has a curved, posterior end for grasping the female during copulation.
These differences in size and morphology are examples of sexual dimorphisms.
Why do you think sexes of Ascaris differ in size?
B. Ascaris, internal morphology (Figure 6)
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 159 of 10
Obtain an Ascaris worm from your laboratory TA. Female Ascaris are somewhat
easier to dissect, because their larger size makes it easier to find and identify
various organs. However, you should examine both a dissected male and female
worm, so ask around in lab to find a dissected worm of the opposite sex.
Determine the dorsal surface by locating the anus, which is on the ventral side.
Then, place the animal in a dissecting pan, pinning it at both the head and tail ends,
dorsal side up. Using fine scissors or a scalpel, carefully cut along the midline of
the dorsal surface to expose the internal organs. Pin the body wall back so that
organs are exposed, and submerge your animal in water so that its internal organs
float freely.
Figure 6: External and internal anatomy of A. female and B. male Ascaris (from
Hopkins & Smith, 1997, Introduction to Zoology).
Note the body cavity, which is a false coelom (pseudocoel). How does this
pseudocoel differ from a true coelom? The two, faint lateral stripes are lateral lines
that bear excretory canals which empty into an excretory pore, located anteriorly
on the ventral surface (not visible). Other, fainter longitudinal streaks are bundles
of longitudinal muscle, formed from embryonic mesoderm. There are no circular
muscles. Given the absence of a hard, bony skeleton and circular muscle, how do
you think a nematode moves?
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 160 of 10
The straight, tubular digestive system for the most part is undifferentiated (why?)
and consists of a mouth, pharynx, intestine, and anus.
The most conspicuous organs in the pseudocoel are the tubular reproductive
organs. Nematodes are very prolific, and females of some species may shed
thousands of eggs daily. Carefully uncoil the reproductive organs, which are Yshaped. The vagina is located at the base of the Y, and the two arms are the uteri.
Each uterus connects to an oviduct, which in turn connects to an ovary. The uterus,
oviduct, and ovary are continuous and have no obvious demarcations between
them, although the uterus tends to be slightly larger in diameter.
3) Phylum Annelida
The phylum Annelida includes approximately 15,000 marine, freshwater,
terrestrial, and parasitic species. It is the archetypal 'wormy' phyla, with the
majority of forms possessing a long, thin shape. The long shape is attained in
annelids by metameric segmentation, a linear repetition of body parts and organs.
Segmentation has enabled annelids to become particularly adept at a particular type
of locomotion, burrowing. In addition to segments, other annelid features include:
1) A triploblastic, bilaterally-symmetric body plan with a true coelom; that is, their
body cavity is completely lined by mesodermally-derived tissue (the peritoneum).
2) The fluid-filled coelom functions as a hydrostatic skeleton.
3) A closed circulatory system with dorsal and ventral blood vessels, with one to
many "hearts"; often with hemoglobin as a respiratory pigment.
4) A complete, one-way, digestive tract, with a separate mouth and anus.
The phylum is divided into three classes, two of which are characterized by
tiny bristles (setae) in their body walls:
Class Polychaeta (= many setae), marine species such as sandworms that usually
possess fleshy, lateral extensions (parapodia) from their body wall.
Class Oligochaeta (= few setae), freshwater and terrestrial species (e.g.,
earthworms).
Class Hirudinea, leeches, which lack setae and move in an inch-worm fashion
using anterior and posterior suckers, or swim via undulations.
Earthworm dissection: Obtain a preserved specimen of the earthworm
(Lumbricus) for dissection. Identify the dorsal and ventral surfaces. Make an
incision on the dorsal surface from the prostomium (mouth) to the middle of the
body. Carefully cut and pin back the skin to expose the internal anatomy. Use
Figure 8 to identify the structures listed below, and consider the basic function of
each structure as you examine it.
You should be able to identify the following structures on a dissected earthworm:
prostomium
crop
gizzard
pharynx
intestine
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 161 of 10
esophagus
Figure 8. Internal anatomy of the earthworm, Lumbricus (from Wallace, et al.,
1989; Invertebrate Zoology)
Hirudo, preserved specimen (demonstration), ectoparasite
Observe the specimens of Hirudo, a leech representative of the class
Hirudinea. Leeches probably evolved from oligochaetes, and are the most
specialized of annelids. Some leeches are predaceous, but most are external
parasites of other animals, and have several adaptations for a parasitic lifestyle.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 162 of 10
Their body is dorso-ventrally flattened, and the first and last segments are modified
to form suckers. Why is a flat shape useful for ectoparasites? Can you think of any
arthropod parasites that have flat shapes? Except in primitive species, the internal
segmentation has been lost. Consequently, the movement of leeches differs
somewhat from other annelids, and depends on the use of suckers for attachment to
the substrate. Given what you know about locomotion in earthworms, how do you
think a leech uses its suckers to move about? The mouth of the leech has toothed
jaws, which it uses to make an incision in its host to feed on its blood. An
anticoagulant, hirudin, secreted into the wound keeps the host's blood flowing.
What arthropods might benefit from having such an anticoagulant? Like
oligochaetes, leeches are hermaphroditic, bearing both male and female
reproductive organs.
Anterior sucker/mouth
Intestine
DIGESTIVE SYSTEM (FOOD PROCESSING)
Movement, coordination and sensing things all require energy, so organisms
need ways to capture and process energy from their environment. In some
organisms (plants), this is a matter of directly harnessing the sun's energy to
convert carbon to glucose (photosynthesis), but in many other organisms, locating
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 163 of 10
and immobilizing prey to obtain energy is a complex process. Once animals have
located and/or subdued their food, that food needs to get broken down into smaller
bits, nutrients like glucose need to get absorbed into the body system, and waste
products must be eliminated. This requires some sort of digestive system.
You probably know the story in mammals (like you) - you break apart the
food with your teeth and enzymes (amylase) found in your saliva, then you
swallow the smaller bits, and enzymes in your stomach and small intestine break it
down further. Although substances like alcohol and caffeine can be absorbed
across the stomach lining (hence the buzz when consumed on an empty stomach),
the site of most absorption of nutrients occurs in the small intestine. The surface of
the small intestine is lined with villi, projections into the lumen of the intestine that
dramatically increase the surface area for digestion. The small intestine has three
specialized regions: 1) the duodenum, where most digestion occurs, 2) the
jejunum, and 3) the ileum, where (with the jejunum) 90% of the absorption of
nutrients occurs. From the small intestine, waste passes into the colon where water
and ions are absorbed into the body, and undigested wastes are eliminated through
the rectum and anus.
So, what happens in other organisms that are not you? How do invertebrates go
about the processes of acquiring food, where do they digest it, and where do they
absorb it? Knowing what they need to do, think about what types of materials each
organism consumes, and try to locate the likely places that these processes occur.
Refer back to Figure 8 and to your dissected earthworm to answer these questions
for the free-living earthworm.
Animal Diversity and Organ Systems Lab I: Worms
The questions that follow will more specifically guide your analyses. The list of
questions is not exhaustive, but should direct your attention to specific key points
of the worm systems that you will see today. Some questions will require that you
synthesize information from lectures, your book and the lab.
Questions on Worm Phylogeny:
1. What are the major or features of the platyhelminthes?
2. What are the major taxonomic divisions in the phylum Platyhelminthes?
3. What are the major features of the nematodes?
4. What are the major features of the annelids?
5. What are the major taxonomic divisions in the phylum Annelida?
6. If comparing two organisms, what characteristics do they share because of
homology (history)? What do they share because of convergent evolution?
7. How are parasitic worms similar and different from their free-living relatives?
What structures have they lost? What structures/organs are expanded?
8. Are there features that are common between the ecto- and endoparasites that you
observed? What are the differences between these types of parasites?
9. Why is a flat shape useful for ectoparasites? Can you think of any arthropod
parasites that have flat shapes?
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 164 of 10
Questions on Organ Systems Anatomy and Physiology:
Digestive System:
1. What are the three major processes that occur in the digestive system?
2. How does an earthworm process its food? What structure manually breaks down
food particles?
3. What is the function of the intestine in all animals? What are the implications of
increasing the length (and/or surface area) of the small intestine? How is the
surface area of the earthworm intestine increased?
4. What structural feature(s) does the gastrovascular cavity of Dugesia share with
the intestine of the earthworm?
5. Why is the digestive tract of Ascaris so unspecialized and why don't tapeworms
have one at all?
6. The mouth of the leech has toothed jaws, which it uses to make an incision in its
host to feed on its blood. An anticoagulant, hirudin, secreted into the wound keeps
the host's blood flowing. What arthropods might benefit from having such an
anticoagulant?
Laboratory #13 Structure and origin of the excretory system of flat, round and
annelids. Circulatory and respiratory systems nemertine and annelids.
1. Features of the structure of the excretory system of worms.
2. Role covers breathing worms.
3. Gills.
4.Circulatory and respiratory systems nemertine and annelids.
Laboratory #13. Structure and origin of the excretory system of flat, round and
annelids. Circulatory and respiratory systems nemertine and annelids.
1. Features of the structure of the excretory system of worms.
2. Role covers breathing worms.
3. Gills.
4.Circulatory and respiratory systems nemertine and annelids.
topic, , methodical recommendations, questions for self-protection and
laboratory classes
Objectives:
■ Understand the taxonomic relationships and major features of the worm phyla,
Platyhelminthes, Nematoda and Annelida
■ Learn the excretory system of Dugesia, Clonorchis, and Ascaris and become
familiar with the internal features of this specimens
Introduction:
The excretory system of the worm is called a nepheridia. In this system, a
ciliated tunnel is located that ends in a bladder-like sac. This sac fills with the
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 165 of 10
waste fluid of the worm which later opens to be released through a pore to its
surrounding.
Excretory System Function
1. Collect water and filter body fluids.
2. Remove and concentrate waste products from body fluids and return other
substances to body fluids as necessary for homeostasis.
3. Eliminate excretory products from the body.
Many invertebrates such as flatworms use a nephridium (nephridium - the
excretory organ in þatworms and other invertebrates; a blind-ended tubule that
expels waste through an excretory pore) as their excretory organ. At the end of
each blind tubule of the nephridium is a ciliated flame cell (flame cell - a
specialized cell at the blind end of a nephridium that Þlters body þuids). As fluid
passes down the tubule, solutes are reabsorbed and returned to the body fluids.
Excretory system of a flatworm. Image from Purves et al., Life: The Science of
Biology, 4th Edition, by Sinauer Associates (www.sinauer.com) and WH Freeman
(www.whfreeman.com), used with permission.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 166 of 10
Excretory system of an earthworm. Image from Purves et al., Life: The Science of
Biology, 4th Edition, by Sinauer Associates (www.sinauer.com) and WH Freeman
(www.whfreeman.com), used with permission.
1) Phylum Platyhelminthes
The excretory system consists of protonephridia. These are branching canals
ending in so-called flame cells—hollow cells with bundles of constantly moving
cilia.
Flukes, like other flatworms, have protonephridia, and there is typically a
pair of longitudinal collecting ducts. There may be two anterior, dorsolateral
nephndiopores (in Monogenea) or a single posterior bladder and nephridiopore (in
Trematoda. In the ectoparasites, the protonephridia are probably only
osmoregulatory in function. The function of the protonephridia in en-doparasites is
still uncertain.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 167 of 10
Figure 4: Schematic of the trematode, Clonorchis (from Hopkins & Smith, 1997,
Introduction to Zoology).
Type Nemathelminthes.
Excretion
Protonephridia are absent in all nematodes and) patently disappeared with
the ancestral mere of the class. Some nematodes have no special excretory system,
but many do possess a peculiar system of gland cells, with or without tubules, that
has some excretory function. In the class Adenophorea, which includes most
marine and freshwater nematodes, there is usually one large gland cell, called a
renette gland (Fig. 9-19A), located ventrally in the pseudocoel near the pharynx.
The gland cell is provided with a necklike duct that opens ventrally on the midline
as an excretory pore.
All members of the class Secernentea, which includes many terrestrial
species, have a more specialized tubular system, still composed of only a few cells.
Three long canals are arranged to form an H (Fig. 9-19B). Two are lateral and
extend inside the lateral longitudinal cords. The two lateral cauls are connected by
a single transverse canal, from which a short, common, excretory canal leads to the
excretory pore, located ventrally on the midline. In many nematodes, that part of
each lateral canal anterior to the transverse canal has disappeared, so the system is
shaped like a horseshoe; in others the tubules on one side have been lost, so the
system is asymmetrical.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 168 of 10
The excretory gland cell or tubules are known to eliminate foreign
substances, but may have other functions as well. Ammonia is the principal
nitrogenous waste of nematodes and is removed through the body wall and
eliminated from the digestive system along with the indigestible residues.
Type Annelida.
Class Polychaeta.
METANEPHRIDIA
The most common type of excretory organ among coelomate animals is a
metanephridium. In contrast to the blind protonephridial tubule, a metanephridial
tubule opens internally into the coelom. The opening is often funnel-like and
clothed with ciliated perito-num, in which case it is called a nephrostome. In
imsegmented coelomates there may be one nephrite or one to several pairs of
metanephridia; in segmented groups, such as the annelids, the metanephridia are
serially repeated, one pair per segment.
In general, a metanephridium processes coelomic hid. Blood filtrate passes
into the coelom at various sites of filtration, depending on the species. For example, in a mollusk part of the heart wall is the major ate of filtration and is
composed of podocytes, cells with finger-like processes that interdigitate [Fig. 1190]. The slits between processes are the sites of titration. Podocytes are found at
the filtration sites of many animals, e.g., the glomeruli of the vertebrate kidney
Coelomic fluid, derived from blood filtrate, passes through the nephrostome into
the ciliated nephridial tubule. Here it becomes modified by selective reab-sorption
and secretion, and the product is finally expelled through the nephridiopore as
urine. The extent of tubular secretion and reabsorption depends in pert on the
environment in which the animal lives, i.e., whether it is an osmoconformer or
osmoregula-tor. The tubule wall is correspondingly specialized and provided with
a vascular backing.
Excretion
Polychaete excretory organs are either protonephridia or metanephridia (Box
10-2). In primitive polychaetes there is one pair of nephridia per segment, but
reduction to few or even one pair for the entire worm has occurred in some
families. The anterior end of the nephridial tubule is located in the coelom of the
segment immediately anterior to that from which the nephridiopore opens (Fig. 102). The tubule penetrates the posterior septum of the segment, extends into the next
segment, where it may be coiled, and then opens to the exterior in the region of the
neuropodium. Both the preseptal portion of the nephridium and the pos-tseptal
tubule are covered by a reflected layer of peritoneum from the septum.
Protonephridia of a type called solenocytes are found in phyllodocids, alciopids,
tomopterids, gly-cerids, nephtyids, and a few others. The solenocytes are always
located at the short preseptal end of the nephridium and are bathed by coelomic
fluid. The solenocyte tubules are very slender and delicate and arise from the
nephridial wall in bunches (Fig. 10-37) Each tubule contains a single flagellum,
and the wall is composed of parallel rods connected by the thin lamellae. The latter
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 169 of 10
represent the fenestrations through which fluid passes; this arrangement is
characteristic of other types of protonephridia.
All other polychaetes possess metanephridia, in which the preseptal end of
the nephridium possesses an open, ciliated funnel, the nephrostome, instead of
solenocytes. Typical metanephridia are found in the nereids, where the
nephrostome possesses an outer investment of peritoneum and the interior is
heavily ciliated. The postseptal canal, which extends laell next successive segment,
becomes greatly coded form a mass of tubules, which are enclosed J thin, saclike
covering of peritoneal cells. CoiliJ probably an adaptation that increases the игШ
area for tubular secretion or rcabsorption. TkJ phridiopore opens at the base of the
neuropcdJ on the ventral side. The entire lining of thetutJ is ciliated.
The metanephridia of most other polycbl differ only in minor details (Fig.
10-38) bill display various degrees of regional restncwl the more specialized
families. In the fan worn! where only one pair of functional nephndul main, the
two nephridia join at the midline to Л a single median canal, which extends forwrJ
open through a single nephridiopore on tbet. Excretory waste is deposited directly
ouuide, and fouling of the tube is avoided.
In polychaetes the association of the blood vcs-Klswith the nephridia is
variable. The fan worms HKt the arenicolids lack a well-developed nephridial
blood supply, and the coelomic fluid must be the principal route for waste removal.
In other po-hxhaetes the nephridia are surrounded by a network of vessels. In the
nereids the nephridial blood supply is greater in those species that live in brack-lb
water.
Many polychaetes, particularly nereids, can tolerate low salinities and have
become adapted to life mbrackishsounds and estuaries. The gill (notopo-dullobe)
of Nereis succinea contains cells specially fot absorbing ions. A small number of
species hve in fresh water. The sabellid Manayunkia spe-:wexample, occurs in
enormous numbers in ctrum regions of the Great Lakes, such as around themouth
of the Detroit River. There are a few ter-Шіаі polychaetes, all tropical Indo-Pacific
nereids, which burrow in soil or live in moist litter.
Chloragogen tissue, coelomocytes, and the intestinal wall may play
accessory roles in excretion. Chloragogen tissue is composed of brown or greenish
cells located on the wall of the intestine or on various blood vessels. Chloragogen
tissue, which has been studied much more extensively in earthworms (see p. 316),
is an important center of intermediary metabolism and hemoglobin synthesis.
Class Oligochaeta.
Excretion
The adult oligochaete excretory organs are metanephridia, and typically,
there is one pair per segment except at the extreme anterior and posterior ends. In
the segment following the nephrostome, the tubule is greatly coiled, and in some
species, such as Lumbricus, there are several separate groups of loops or coils.
Before the nephridial tubule opens to the outside, it is sometimes dilated to form a
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 170 of 10
bladder. The nephridiopores are usually located on the ventrolateral surfaces of
each segment.
In contrast to the majority of oligochaetes, which possess in each segment a
single, typical pair of nephridia called holonephridia, many earthworms of the
families Megascolecidae and Glos-soscolecidae are peculiar in possessing
additional nephridia, which are multiple or branched. Either typical or modified
nephridia may open to the outside through nephridiopores, or they may open into
various parts of the digestive tract, in which case they are termed enteronephric. A
single worm may possess a number of different types of these nephridia, each
being restricted to certain parts of the body.
Earthworms excrete urea, but they are less perfectly ureotelic than are other
terrestrial animals. Although urea is present in the urine of Lumbricus and other
earthworms and although the level of urea depends on the condition of the worm
and the environmental situation, ammonia remains an important excretory product.
Salt and water balance, which is of particular importance in freshwater and
terrestrial environments, is regulated in part by the nephridia (Fig. 10-54B). The
urine of both terrestrial and freshwater species is hypoosmotic, and considerable
reabsorption of salts must take place as fluid passes through the nephridial tubule.
Some salts are also actively picked up by the skin.
In the terrestrial earthworms water absorption and loss occur largely through
the skin. Under normal conditions of adequate water supply, the nephridia excrete
a copious hypoosmotic urine. It is not certain whether reabsorption by the ordinary
nephridia is of importance in water conservation, but the enteronephric nephridia
do appear to represent an adaptation for the retention of water. It passing the urine
into the digestive tract, muchl the remaining water can be reabsorbed as it goes
through the intestine. Worms with enteronepmi systems can tolerate much drier
soils or do J have to burrow so deeply during dry periods.
A few aquatic oligochaete species are capabled encystment during
unfavorable environmenrd conditions. The worm secretes a tough, mucca covering
that forms the cyst wall. Some specie form summer cysts for protection against
desicr*! tion; others form winter cysts when thewatertet perature becomes low.
During dry seasons or during the winter, eaii worms migrate to deeper levels of the
soil, dm 3 meters in the case of certain Indian species.Ц moving to deeper levels,
an earthworm often » dergoes a period of quiescence and in drypeJ may lose as
much as 70 per cent of its water.№ ance is restored and activity resumed as soon»
water is again available.
Class Hirudinea.
Excretion
Leech contain 10 to 17 pairs of metanephridia, ill the middle third of the
body, one pair segment. As a result of the coelom ton and the loss of septa in the
leech body, ihndial tubules arc embedded in connective the nephrostomcs project
into the coc-c channels. Each nephrostome opens into a itedcapsule.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 171 of 10
In most leeches the cavities of the capsule and idial canal do not connect, and the
two of the nephridium may even have lost ictural connection. Many branching,
intra-ilar canals drain into the nephridial canal, rpens to the outside through the
ventrola-nephndiopore. Secretion into the intracellular iculiisthe initial source of
nephridial fluid, Btfleunne is very hypoosmotic to the blood, in-inung reabsorption
of salts. The nephridia are important organs of osmoregulation (Haupt,
The function of the nephridial capsules is be-Btdtobe the production of
coelomocytes. The coelomocytes are phagocytic and engulf particulate matter, but
the eventual fate of the waste-laden cells is not certain. They may migrate to the
epidermis or to the epithelium of the digestive tract. Particulate waste is also
picked up by botryoidal and vasofibrous tissue of the hirudinid leeches and by
pigmented and coelomic epithelial cells of glos-siphoniids and piscicolids.
Methodical recommendations
Phylum Plathelminthes. The Water-vascular System of Flatworms. A small
opening will be found at the posterior end of the body from which a duct passes
forward in a median position to a point a little posterior to the median sucker. Here
it divides and sends a branch on either side of the worm to near the anterior end.
Make a drawing showing the above structures as far as you have seen them.
The Water-vascular System of Cestoda.
Look for transparent tubes coiling about in the scolex and its suckers. Compress
the specimen by drawing off as much water as possible with filter-paper, and look
again for the transparent tubes. These are portions of the water-vascular system.
Recall the description of this system given in the lecture or in text-books. The finer
branches which lead from the main trunks are difficult to identify with certainty,
but by using the high power of your microscope, and focusing just below the
surface in the more transparent portions of the scolex, the flame cells may easily be
seen. The " flame " appears like a short, thick whip lost in continual vibration. Find
such flames and watch them carefully. If
not found at once, lot the preparation stand and examine in about half an hour. In
the older preparation they are frequently easier to find.
Make a drawing showing the above structures as far as you have seen them.
Use the following labels to identify the photographs. You may have to use a label
more than once, and some labels may not be appropriate for the photographs.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 172 of 10
Specimens: A- Opisthorchis (Chlonochis) B- Diplydium C- Obelia DParagonimus E- Dugesia Labels
1) Excretory canal
Phylum Nemathelminthes.
Nephridia of Annelida
Earthworms (annelids) and some other invertebrates, such as arthropods and
mollusks, have slightly-more-evolved excretory structures called nephridia . A pair
of nephridia is present on each segment of the earthworm. They are similar to
flame cells in that they have tubules with cilia and function like a kidney to remove
wastes, but they often open to the exterior of the organism. The ciliated tubules
filter fluid from the body cavity and carry waste, including excess ions, through
openings called nephrostomes. From the nephrostomes, excretion occurs through a
pore called the nephridiopore. A nephridium is more evolved than a flame cell in
that it has a system for reabsorption of some useful waste products, such as
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 173 of 10
metabolites and ions, by a capillary network before excretion (unlike planaria that
can only reabsorb useful metabolites after excretion).
Questions on Organ Systems Anatomy and Physiology:
Digestive System:
1. What are the three major processes that occur in the digestive system?
2. How does an earthworm process its food? What structure manually breaks down
food particles?
3. What is the function of the intestine in all animals? What are the implications of
increasing the length (and/or surface area) of the small intestine? How is the
surface area of the earthworm intestine increased?
4. What structural feature(s) does the gastrovascular cavity of Dugesia share with
the intestine of the earthworm?
5. Why is the digestive tract of Ascaris so unspecialized and why don't tapeworms
have one at all?
6. The mouth of the leech has toothed jaws, which it uses to make an incision in its
host to feed on its blood. An anticoagulant, hirudin, secreted into the wound keeps
the host's blood flowing. What arthropods might benefit from having such an
anticoagulant?
Laboratory #14 Origin, structure and evolution of the nervous system of flat,
round and annelids.
1. Features of the structure of the nervous system of worms.
2. Types of nervous systems of worms.
Laboratory #15. Origin, structure and evolution of the nervous system of flat,
round and annelids.
3. Features of the structure of the nervous system of worms.
4. Types of nervous systems of worms.
topic, , methodical recommendations, questions for self-protection and laboratory
classes
Supplies
Equipment
Compound microscopes
Dissecting microscopes
Materials
Prepared slides
preserved specimen
Student Prelab Preparation
Before doing this lab, you should read the introduction and sections of the
lab topic.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 174 of 10
You should use your textbook to review the definitions of the following
terms:
clitellum
septum
ventral nerve cord
seminal vesicle
hearts
cerebral ganglia (“brain”)
nephridia
dorsal blood vessel
You should be able to describe in your own words the following concepts:
How a nerve cell functions
Types of nervous systems found in animals
Major regions of the brain
As a result of this review, you most likely have questions about terms,
concepts, or how you will do the experiments included in this lab.
Objectives
1. To observe the microscopic structures of nervous system
2. To learn the anatomy of the worms
3. To determine experimentally some of the properties nervous system
Introduction:
During this week of our animal diversity survey, we will study three worm
phyla. All of the phyla of worms that we will examine - the annelids, the
nematodes, and the platyhelminthes - contain species that are parasites of humans
(not to mention other animals and plants). You may already be familiar with some
of these creatures: you are likely to encounter leeches (an annelid) simply from
wading in a steam or pond, and if you ever had a dog or cat, you probably took it
to the vet at least once to be treated for worms (such as roundworms and
whipworms, both nematodes, and tapeworms, a platyhelminth).
The parasitic worms that you will examine are for the most part eating and
reproducing machines. Consequently, when studying the parasitic worms, take a
good look at their digestive and reproductive systems, and then compare them to
the digestive and reproductive systems of free-living worms (e.g., earthworms).
1) Phylum Platyhelminthes
The Platyhelminthes include free-living flatworms, like the planarians, and
the parasitic tapeworms and flukes. The term flatworm refers to the fact that the
body is dorsoventrally flattened. Flatworms are the first organisms to have tissues
organized into organs and the first to demonstrate bilateral symmetry. Bilateral
symmetry means that one plane passing through the longitudinal axis of an
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 175 of 10
organism divides it into right and left halves that are mirror images. It is
characteristic of active, crawling, or swimming organisms and usually results in
the formation of a distinct head (cephalization) where accumulation of nervous
tissue and sensory structures occurs. This reflects the importance to the organism
of monitoring the environment it is meeting
- rather than that through which it has just passed - and results in the presence of
definite anterior and posterior ends. The Platyhelminthes and all phyla above them
on the evolutionary tree are bilaterally symmetrical or have evolved from
bilaterally symmetrical ancestors.
In the Platyhelminthes, different tissues cooperate in any given function.
This results in the organ level of organization. Three major sets of organs
characterize the phylum. The excretory system consists of flame cells and their
associated ducts. The nervous system consists of a pair of anterior ganglia,
usually with two nerve cords winning the length of the organism. Nerve cords are
interconnected by transverse nerves to form a ladder-like structure. The digestive
tract is incomplete (a single opening serves for ingestion of food and elimination of
wastes).
The Platyhelminthes are triloblastic and acoelomate. There are three
primary germ layers: ectoderm,endoderm, and mesoderm. As with the Cnidaria,
the ectoderm gives rise to the outer epithelium, and the endoderm gives rise to the
lining of the gut tract The third germ layer, the mesoderm, gives rise to the tissue
between the ectoderm and the endoderm, including muscle, excretory structures,
and undifferentiated cells referred to as parenchyma. The term acoelomate refers
to the fact that them is no body cavity (fluid-filled space) between any of the
primary germ layers.
The phylum is divided into four classes:
Class Turbellaria, free-living marine, freshwater, and terrestrial flatworms. Class
Trematoda, parasitic internal flukes
Class Cestoda, parasitic tapeworms
Class Monogenea, parasitic external flukes
Methodical Recommendations:
1. Specimens of Platyhelminthes
We will examine live speciemens (Dugesia) and microscope slides (Dugesia,
Clonorchis, Taenia) representative of free-living and parasitic platyhelminthes.
A) Dugesia, Class Turbellaria, live specimen.
Obtain a live Dugesia flatworm by sucking it up from the side or bottom of a
glass jar using a medicine dropper. Place the specimen in a small Petri dish,
making sure that it is completely covered by pond water, and examine it under
your dissecting scope.
Dugesia is a common turbellarian (= planarian) that resides in freshwater
steams and ponds. Note your animal's shape, pigmentation, and mode of
locomotion. Dugesia, as well as most free-living flatworms, move over surfaces by
means of cilia on their ventral surface. Note the pigmented eye spots, or ocelli,
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 176 of 10
located on the triangular "head" of the animal. These eye spots are sensitive only to
light and dark, and are unable to resolve images. On either side of the eye spots are
lateral lobes which serve as chemosensory organs. Cover your culture dish (top
and sides) with a piece of aluminum foil, and place the dish on a dark background
with a microscope light shining on it. After 5 to 10 minutes, remove the foil and
observe where your animal is relative to the light. Is your animal positively or
negatively attracted to light (= phototactic)? How might this behavior be adaptive
for the animal in its natural environment?
B) Dugesia, microscope slide (Figure 2).
1. Observe a prepared whole mount of Dugesia under low power of your
compound microscope. You should see the eye spots and the "brain", nerve cord.
2.
The cerebral ganglia, a bilobed structure beneath the eye-spots, that appears
as a slightly lighter area.
3.
From the cerebral ganglia two longitudinal nerve cords pass backward, and
several smaller nerves pass off in front. Ex¬amine the specimen by reflected light,
looking particularly at the nervous system and pharynx. What relation have the
nerve cords behind?
4.
With the high power and good light, look for the water-vascular tubules. The
region anterior to the cerebral ganglia is a favorable place. They form a clear,
branching tracery, a little lighter than the surrounding tissue. The flicker of the
flame cells can usually be seen, but they may be more easily seen in
Crossobolhrium Examine chart and text-book figures of the water-vascular system.
Make a good-sized drawing of a worm, showing the above points.
Figure 2: Dugesia, whole mount (from Stamps, Phillips & Crowe. The laboratory:
a place to do science, 3rd ed.)
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 177 of 10
C) Clonorchis, Class Trematoda, preserved specimen and microscope slide
(Figure 3).
Clonorchis sinensis, the human liver fluke, is a parasitic trematode found in
the bile ducts of humans. Like most parasitic worms, the life cycle of C. sinensis is
extremely complex and involves several hosts. The adult worm sheds eggs into the
bile ducts of its human host, which eventually reach the small intestine and are
passed with feces. If the eggs are ingested by the proper species of aquatic snail,
they hatch into larvae that then progress through a series of asexual stages,
culminating in an infective larval stage known as cercariae. The cercariae are
ciliated, and have a tail for swimming. They pass out of the snail, and then briefly
swim about in the water until they encounter a fish. Then the cercariae penetrate
the muscles of the fish, lose their tails, and remain encysted until the fish is eaten
by the definitive (= final host). These encysted larvae are freed in the human small
intestine after consumption of improperly prepared fish. The immature flukes
migrate through the bile duct and its tributaries throughout the liver, where they
develop into adult worms. If untreated, an infection by Clonorchis can lead to
enlargement and cirrhosis of the liver.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 178 of 10
Figure 3: Photograph of Clonorchis sinensis, with major features identified (from
Pechenik 1991, Biology of the Invertebrates).
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 179 of 10
Figure 4: Schematic of the trematode, Clonorchis (from Hopkins & Smith, 1997,
Introduction to Zoology).
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 180 of 10
D) Taenia, Class Cestoda, preserved specimen and microscope slide (Figure
5).
Observe a prepared slide of Taenia under low power of your compound
microscope. Your specimen, Taenia pisiformis, is a tapeworm of carnivores
(notably, dogs), and closely resembles T. solium and T. saginata, common
parasites of humans contracted by eating poorly prepared beef or pork,
respectively. Tapeworms share many features with flukes, including an outer
cuticle, attachment structures, expansive reproductive organs, and complex life
cycles involving intermediate hosts. Unlike flukes, however, tapeworms lack a
mouth and gastrovascular cavity, a consequence of their life in vertebrate organs of
high nutritional activity (i.e., the small intestine). Bathed by food in their host's
intestine, they absorb predigested nutrients across their body surface via diffusion
and possibly, active transport.
The body of a tapeworm is divided into four main regions. A small scolex
("head") bears suckers and an elevated rostellum with curved hooks; the suckers
are used for attachment to the host's organs. Immediately posterior to the scolex is
a "neck" that produces many proglottids ("segments") by asexual budding. Each
proglottid is potentially a complete reproductive unit containing by male and
female reproductive organs (i.e., each is hermaphroditic). Why might
hermaphroditism be especially advantageous for an internal parasite?
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 181 of 10
The second region consists of small, immature proglottids nearest to the
neck and scolex.
The third region, or mid-section, consists of mature proglottids, each with
well-developed male and female reproductive organs. These proglottids engage in
internal, cross fertilization. In a mature proglottid, locate the lateral genital pore
that contains both a thin, tubular vagina and a stouter vas deferens. Trace the
vagina posteriorly and note that it passes between two ovaries and terminates at a
shell gland anterior to a yolk gland. Eggs are fertilized and "yolked" before passing
anteriorly into a sac-like uterus. The male reproductive system consists of
numerous small, round testes, each with a tiny tubule that connects to a single vas
deferens, which transports sperm to the genital pore.
The fourth and posterior region of the tapeworm consists of gravid (= "pregnant")
proglottids. In gravid proglottids, most of the gonads are atrophied, leaving only an
enlarged uterus packed with eggs. These gravid proglottids eventually break off
from the body of the adult worm, and pass out of the digestive tract in the host's
feces. When a small mammal, such as a rabbit, ingests a proglottid or eggs, the
eggs hatch into larvae that then bore through the intestinal wall and then move
through the circulatory system where they eventually become encysted in muscle
tissue. When the rabbit is eaten by a dog, the encysted larvae are released, and
develop into adult worms. As can be seen from the specimen on display,
tapeworms can be quite large: T. solium, a parasite of the human intestine, can
reach a length of 10 feet!
2) Phylum Nematoda
Nematodes are probably the most abundant and ubiquitous animals on earth,
having invaded virtually every habitat. Most of the approximately 10,000 species
of nematodes are free-living, but many are parasites of animals, including humans.
Trichinella spiralis, for example, is contracted by eating insufficiently cooked pork.
The adult worms develop in the human intestine, releasing larvae which move
through the lymphatic system, eventually ending up in muscle tissues where they
encyst. Other nasty nematode parasites of humans include Necator americanus
(hookworms) and Wuchereria, which results in elephantiasis. Nematodes also are
parasites of plants and can cause enormous crop damage; as a result, some large
universities have departments of plant pathology devoted to the study of plant
pathogenic nematodes.
Noteworthy characteristics of nematodes are:
1) they are triploblastic.
2) they have a pseudocoelom, a cavity incompletely lined by mesodermallyderived tissue.
3) the fluid-filled pseudocoelom functions as a hydrostatic skeleton.
4) they have a complete, one-way digestive tract, having both a mouth and an anus.
5) they have a non-living, protective cuticle covering their bodies.
Specimens of Nematodes
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 182 of 10
We will examine preserved specimens of Ascaris lumbricoides, commonly
known as the roundworm, an intestinal parasite of humans. Humans contract
Ascaris by ingesting eggs from the soil. Once ingested, the eggs hatch, releasing
larvae. The larvae bore through the small intestine and migrate via the venous and
lymphatic systems to the lungs. There the larvae continue to grow, and pass
through several larval stages. After a few weeks, the larvae are coughed-up,
literally, and then swallowed, where they develop into mature adults in the small
intestine.
A. Ascaris, external morphology (Figure 6)
Examine preserved specimens of male and female ascarids. The male is
smaller, and has a curved, posterior end for grasping the female during copulation.
These differences in size and morphology are examples of sexual dimorphisms.
Why do you think sexes of Ascaris differ in size?
B. Ascaris, internal morphology (Figure 6)
Obtain an Ascaris worm from your laboratory TA. Female Ascaris are somewhat
easier to dissect, because their larger size makes it easier to find and identify
various organs. However, you should examine both a dissected male and female
worm, so ask around in lab to find a dissected worm of the opposite sex.
Determine the dorsal surface by locating the anus, which is on the ventral side.
Then, place the animal in a dissecting pan, pinning it at both the head and tail ends,
dorsal side up. Using fine scissors or a scalpel, carefully cut along the midline of
the dorsal surface to expose the internal organs. Pin the body wall back so that
organs are exposed, and submerge your animal in water so that its internal organs
float freely.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 183 of 10
Figure 6: External and internal anatomy of A. female and B. male Ascaris (from
Hopkins & Smith, 1997, Introduction to Zoology).
Note the body cavity, which is a false coelom (pseudocoel). How does this
pseudocoel differ from a true coelom? The two, faint lateral stripes are lateral lines
that bear excretory canals which empty into an excretory pore, located anteriorly
on the ventral surface (not visible). Other, fainter longitudinal streaks are bundles
of longitudinal muscle, formed from embryonic mesoderm. There are no circular
muscles. Given the absence of a hard, bony skeleton and circular muscle, how do
you think a nematode moves?
The straight, tubular digestive system for the most part is undifferentiated (why?)
and consists of a mouth, pharynx, intestine, and anus.
The most conspicuous organs in the pseudocoel are the tubular reproductive
organs. Nematodes are very prolific, and females of some species may shed
thousands of eggs daily. Carefully uncoil the reproductive organs, which are Yshaped. The vagina is located at the base of the Y, and the two arms are the uteri.
Each uterus connects to an oviduct, which in turn connects to an ovary. The uterus,
oviduct, and ovary are continuous and have no obvious demarcations between
them, although the uterus tends to be slightly larger in diameter.
3) Phylum Annelida
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 184 of 10
The phylum Annelida includes approximately 15,000 marine, freshwater,
terrestrial, and parasitic species. It is the archetypal 'wormy' phyla, with the
majority of forms possessing a long, thin shape. The long shape is attained in
annelids by metameric segmentation, a linear repetition of body parts and organs.
Segmentation has enabled annelids to become particularly adept at a particular type
of locomotion, burrowing. In addition to segments, other annelid features include:
1) A triploblastic, bilaterally-symmetric body plan with a true coelom; that is, their
body cavity is completely lined by mesodermally-derived tissue (the peritoneum).
2) The fluid-filled coelom functions as a hydrostatic skeleton.
3) A closed circulatory system with dorsal and ventral blood vessels, with one to
many "hearts"; often with hemoglobin as a respiratory pigment.
4) A nervous system including a cerebral ganglion (= brain).
5) An excretory system consisting of nephridia.
The phylum is divided into three classes, two of which are characterized by
tiny bristles (setae) in their body walls:
Class Polychaeta (= many setae), marine species such as sandworms that usually
possess fleshy, lateral extensions (parapodia) from their body wall.
Class Oligochaeta (= few setae), freshwater and terrestrial species (e.g.,
earthworms).
Class Hirudinea, leeches, which lack setae and move in an inch-worm fashion
using anterior and posterior suckers, or swim via undulations.
Earthworm dissection: Obtain a preserved specimen of the earthworm
(Lumbricus) for dissection. Identify the dorsal and ventral surfaces. Make an
incision on the dorsal surface from the prostomium (mouth) to the middle of the
body. Carefully cut and pin back the skin to expose the internal anatomy. Use
Figure 8 to identify the structures listed below, and consider the basic function of
each structure as you examine it.
You should be able to identify the following structures on a dissected earthworm:
clitellum
septum
ventral nerve cord
seminal vesicle
hearts
cerebral ganglia (“brain”)
nephridia
dorsal blood vessel
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 185 of 10
Figure 8. Internal anatomy of the earthworm, Lumbricus (from Wallace, et al.,
1989; Invertebrate Zoology)
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 186 of 10
Hirudo, preserved specimen (demonstration), ectoparasite
Observe the specimens of Hirudo, a leech representative of the class
Hirudinea. Leeches probably evolved from oligochaetes, and are the most
specialized of annelids. Some leeches are predaceous, but most are external
parasites of other animals, and have several adaptations for a parasitic lifestyle.
Their body is dorso-ventrally flattened, and the first and last segments are modified
to form suckers. Why is a flat shape useful for ectoparasites? Can you think of any
arthropod parasites that have flat shapes? Except in primitive species, the internal
segmentation has been lost. Consequently, the movement of leeches differs
somewhat from other annelids, and depends on the use of suckers for attachment to
the substrate. Given what you know about locomotion in earthworms, how do you
think a leech uses its suckers to move about? The mouth of the leech has toothed
jaws, which it uses to make an incision in its host to feed on its blood. An
anticoagulant, hirudin, secreted into the wound keeps the host's blood flowing.
What arthropods might benefit from having such an anticoagulant? Like
oligochaetes, leeches are hermaphroditic, bearing both male and female
reproductive organs.
Anterior sucker/mouth
Intestine
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 187 of 10
INFORMATION PROCESSING AND SENSORY INPUT
The bodily processes of multicellular organisms need to be coordinated, and
to do this, most animals have a nervous system, which enable them to coordinate
and regulate the activities of different body parts via rapid electrical signaling. The
simplest type of nervous system consists of simple sensors, which receive
information, effectors such as muscles and glands, which carry out responses to
stimuli, and a system of nerves that run between them. Some organisms, like
cnidarians (e.g., Hydra), can exist with a simple network of neurons since their
lives are spent attached to the substratum, and responses to their environment do
not need to be well coordinated.
For animals that move about to locate food or mates, more-sophisticated
sensory detection and coordination of responses is necessary. In these animals, we
see the development of simple to complex brains (or ganglia, which are
accumulations of nerve cell bodies, in the case of invertebrates), well-defined
sensory systems, and developed effectors (like muscles). In addition, the nerves
that run between these components of the nervous system become increasingly
more developed from simple to complex organisms. When you look at the brains
(ganglia) in the specimens over the next several weeks, you should notice where
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 188 of 10
they are located and how complex they are. You should also look at some of the
sensors (such as eyes), the effectors, and the nerves that run between them and the
ganglia in these specimens. In particular, pay attention to where the major nerves
are located (are they on the dorsal side or the ventral side?).
Examples of Invertebrate Nervous Systems:
Brains: In the invertebrates, we see the development of simple brains (ganglia)
that you should try to find today in the earthworm (Lumbricus, Phylum Annelida,
Figure 12) and later in squid and crayfish.
Invertebrate Vision Systems:
In response to their environment, most plants and animals have evolved some
means of detecting light. This detection is generally the consequence of a chemical
change in the organism resulting from the absorption of light energy. Plants
display a general sensitivity to light; that is, they have no specific light-sensitive
organs, yet they are able to detect light and grow or bend toward it. In animals,
there is a tendency toward concentration of light-sensitive cells into specific areas
or organs (e.g., the eyespots in planaria). The development of the eye represents
perhaps the greatest concentration of light-sensitive cells in an organism.
Increasing complexity of association with the nervous system allows the organism
to perceive not only changes in light intensity, but also movement and form.
In today’s lab, examine the eye spots of Dugesia (a planarian worm) as you
test whether Dugesia move towards or away from light. Which other species of
worms have eyes? Why don’t the other species of worms have eyes?
Dorsal
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 189 of 10
Ventral
Figure 12: Head region of the earthworm (Lumbricus), showing the organization
of the nervous system in this region. A major nerve cord runs from the ventral
ganglion down the length of the animal (from Withers, 1992, Comparative Animal
Physiology).
Invertebrate Vision Systems:
In response to their environment, most plants and animals have evolved some
means of detecting light. This detection is generally the consequence of a chemical
change in the organism resulting from the absorption of light energy. Plants
display a general sensitivity to light; that is, they have no specific light-sensitive
organs, yet they are able to detect light and grow or bend toward it. In animals,
there is a tendency toward concentration of light-sensitive cells into specific areas
or organs (e.g., the eyespots in planaria). The development of the eye represents
perhaps the greatest concentration of light-sensitive cells in an organism.
Increasing complexity of association with the nervous system allows the organism
to perceive not only changes in light intensity, but also movement and form.
Questions on Organ Systems Anatomy and Physiology:
Digestive System:
1. What are the three major processes that occur in the digestive system?
2. How does an earthworm process its food? What structure manually breaks down
food particles?
3. What is the function of the intestine in all animals? What are the implications of
increasing the length (and/or surface area) of the small intestine? How is the
surface area of the earthworm intestine increased?
CIRCULATORY SYSTEM (MOVEMENT OF BODY FLUIDS)
Textbook Reference Pages: pp. 1045-1047 (middle)
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 190 of 10
Of course, without a means of distributing both the food (glucose) and the gases
(O2 and CO2) throughout the body, e.g., without a circulatory system, having
gone to the trouble of finding a meal is useless. A circulatory system is essential in
the transport of gases, nutrients, and hormones, as well as critical to aintaining
homeostasis and protection of the body from infection. In lab, we will focus on the
importance of the circulatory system for transport.
Now that you know the functions of the circulatory system, what are the major
components of the system? A true circulatory system consists of one (or more)
pump(s), blood vessels and fluids. Circulatory systems are of two types: open or
closed (you should understand the meaning of those terms). Simpler organisms
such as planaria have a gastrovascular cavity to distribute materials to different
body parts. Earthworms and squid have closed circulatory systems, whereas
crayfish have open systems. Thus, you should think about the puzzle of why
certain organisms may have lost a closed circulatory system over evolutionary
time.
You will not have the opportunity to look at the cellular components of blood in
lab, but be sure to look at the invertebrate hearts and vessels, and think about the
issues raised above.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 191 of 10
Earthworm Circulatory System (Figure 13):
Figure 13: The closed circulatory system of an oligochaete annelid (Lumbricus)
has a dorsal and
ventral vessel and capillary beds in the gut, viscera, and skin; arrows indicate
direction of
blood flow (from Withers, 1992, Comparative Animal Physiology)
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 192 of 10
Laboratory #15 The structure of sense organs in different groups of worms.
1. Olfactory and chemical senses.
2. Tactile organs.
3. The organs of taste.
4.Organs of vision.
Laboratory #16 Evolution and structure of the reproductive system of flat, round
and annelids.
1. Features of the structure of the reproductive system of worms.
2. Sexual and asexual reproduction of worms.
3. Hermaphroditism.
Laboratory #17 Structure of larvae and life cycles of flat, round and annelids.
1. Basic and intermediate host.
2. Structure of larval worms.
3.Life cycles of worms.
Laboratory #18 General characteristics of the type of Arthropods
1. General characteristics of the type.
2. Comparative characteristics of other types.
Laboratory #19 External morphology of crustacean.
1. Avilable crustaceans due to water lifestyle.
1. 2. Function and limb dismemberment of the body of crustaceans
Laboratory #20 Internal morphology of crustacean.
1. Overview of the internal structure of crustaceans.
2. Features of development and types of larvae.
Laboratory #21 Determination of crustacean.
1. Determination of the lower crustaceans.
2.Definition of higher crustaceans.
Laboratory #22 External and internal structure of Chelicerata.
1. Avilable arachnid body parts.
2. Features of the internal structure of arachnids.
Laboratory #23 External and internal structure myriapods.
1. Features of the external structure myriapods.
2. Internal structure of myriapods.
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 193 of 10
Laboratory #24 Features of the external structure of insects. Head and its
appendages.
1. Features of the external structure of insects. Dismemberment of the
body.
2. Types of specialized mouthparts.
Laboratory #25 Features of the external structure of insects. Abdominal and
thoracic appendages. Covers.
1. Types of insect antennae.
2. Types of feet.
3. Types wings.
Laboratory #26 Internal structure of insects.
1. Features of the internal structure of insects.
2. Adaptation of terrestrial animals.
Laboratory #27 Insect reproduction and development.
1. Features of the structure of the reproductive organs of insects.
2.Insect development. Complete and incomplete metamorphosis.
Laboratory #28 Clams external morphology.
1. Features of the external structure of shellfish.
2.Sink.
Laboratory #29 Internal structure of mollusks.
1. Features of the internal structure of mollusks.
2.Comparison of the internal structure of the various classes of molluscs.
Laboratory #30 Deuterostomates. Type Echinoderms.
1. Classes of animals are grouped in type echinoderms: crinoids, starfish, brittle
stars, or zmeehvostki, sea urchins and sea cucumbers and sea cucumbers.
2. Features of the organization, peculiar representatives of all these classes and
characterize the type of echinoderms. . Reproduction and development of
echinoderms: crushing, gastrulation, principal types of larvae and their
metamorphosis, particularly the process of formation of the mesoderm, formation
of secondary cavity.
3.Distribution and lifestyle echinoderms.
4 SELF-STUDY OF THE STUDENT (перечень тем заданий, тесты,
вопросы)
Topic SSS #1 Doctrine of symmetry animals.
Topic SSS #2 The theory of the germ layers. Derivatives ecto-ento and mesoderm.
Topic SSS #3 Ways polymerization and oligomerization (Dogel principle).
EMCD 042-18-35.1.31/ 03-2014
#2 edition from 16.06. 2014
p. 194 of 10
Topic SSS #4 Metamerism. Homonomous heteronomous segmentation and
invertebrates.
Topic SSS #5 Comparative characteristics of the body cavities of invertebrates.
Topic SSS #6 Comparative characteristics of deuterostomes (type Gemihoardate.
7 LITERATURA
7.1.Basic literatura
7.1.1. R.Burns Invertebrate zoology. 1987 by CBS College Publishing.
7.1.2 Догель В.А. Зоология беспозвоночных. -М., 1981.
7.1.3 Курс зоологии. Зоология беспозвоночных. В 2-х томах. -М., 1961,1966.
7.1.4 Иванов А.В. и'др. Большой практикум по зоологии беспозвоночных. Т.
1. -М., 1981.
7.1.5 Иванова-Казас О.М. Курс -сравнительной эмбриологии беспозвоночных
животных. -Л., 1-988.
7.1.6 Фролова Е.Н. и др. Практикум по зоологии беспозвоночных. -М, 1985
7.1.7 Натали В.Ф. Зоология беспозвоночных. - М., 1981.
7.1.8 Зеликман В.А. Практикум по зоологии беспозвоночных. М., 1982.
7.2. Additional literature
7.2.1 Акимушкин И. Причуды природы. Смоленск. Русич, 1999.
7.2.2 Андрианова Н.С. Экология насекомых. – М., 1970.
7.2.3 Жизнь животных. Т. 1-3, М., 1969.
7.2.4 Яхонтов В.В. Экология насекомых. – М., 1969.