* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Congenital abnormalities of aortic artery. Assessment in neonates
Cardiovascular disease wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Drug-eluting stent wikipedia , lookup
Marfan syndrome wikipedia , lookup
Myocardial infarction wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
Turner syndrome wikipedia , lookup
Cardiac surgery wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Coronary artery disease wikipedia , lookup
Aortic stenosis wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
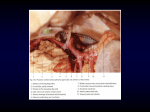
Vascular Original article Congenital abnormalities of aortic artery. Assessment in neonates and early childhood with multislice tomography The multislice angiotomography (MDCTA) presents several properties and is utilized with more frequency every time by childhood cardiovascular surgeons as a chosen method of imaging in the anatomic and pathological scan of different abnormalities of the aortic arch. The multiplanar capacity and three-dimensional reconstructions in particular are easily to interpret, as well as being extremely fast studies, with short anesthetic time and low contrast volume to administer. The objective of the present study has been to review the findings enabled by multislice computer tomography (MDCT) of the aortic arch’s development most frequently found in our institution. MATERIALS AND METHODS Angiotomography studies (CTA) performed between October 2006 and November 2008 to patients who presented congenital abnormalities of aortic artery, were evaluated in a retrospective way. Total patients included in this study were neonates and little children in an age range between 6 days and 11 months. Echocardiography and/ or thorax X ray, together with the symptoms, enabled a presumptive diagnosis (Table 1). 1 2 Servicio de Diagnóstico por Imágenes. Cirugía Cardiovascular Infantil. Fundación Favaloro. Avenida Belgrano 1746. Ciudad de Buenos Aires. República Argentina. Correspondencia: Dr. Diego Haberman: [email protected] The studies were performed with a 64 detector CT (Aquilion , Toshiba Medical Systems, Tokio, Japan) using a software of radiation modulation doses (Sure Exposure) with effective doses between 0.6 to 1.4 mSv. Images taken every 0.5 mm thickness were obtained with a 0.3 mm reconstruction interval, a factor pitch of 0.828 and 0.5 sec rotation tube. Between 1 and 1.5 ml/kg of non ionic intravenous contrast (Iopamiron 370®, Schering) were injected under anesthesia and cardiac monitoring, with a 1.5 to 2.5 ml/sec flow injection pump (Medrad , Stellant). Three-dimension reconstructions were performed in a workstation (Vitrea, Vital Images). All patients were symptomatic and had surgery, which enabled to correlate the information observed by different imaging methods with intra-surgery findings, assumed as certain definitive diagnosis. In 11 cases, the presumptive diagnosis was confirmed by angiotomography. In one patient, the angiocomputed tomography showed a non suspected pathology that was further confirmed by surgery. Embryology review Blood vessels start to develop from the mesoderm that covers the yolk sac at 18 days of the embryo’s age. On day 24th, the cardiovascular system is composed Recibido: abril 2009; aceptado: julio 2009 Received: april 2009; accepted: july 2009 ©SAR-FAARDIT 2009 Página 1 INTRODUCTION Número 3 Congenital abnormalities of aortic artery. Assessment in neonates and early childhodd with multislice tomography In the evaluation of aortic artery congenital abnormalities, the echocardiography and the plain X ray are the traditionally used imaging methods. Multislice angiotomography appears as an important method in diagnosis of these different diseases allowing evaluate these entities in a non invasive, fast and accurate form, giving to cardiovascular surgeons very important information to delineate the surgical strategy. In this article, we review the applications of multislice angiotomography in the evaluation of most frequent congenital anomalies of aorta artery, performed in neonates and early childhood. Key words: Aorta artery. Congenital anomalies. Neonates. Multislice angiotomography. Volumen 73 Abstract Para la evaluación de los defectos congénitos de la arteria aorta, los métodos de imágenes utilizados tradicionalmente son la ecocardiografia y la radiología simple. La angiotomografía computada multicorte aparece como un método de relevancia en el diagnóstico de estas entidades, permitiendo evaluar en forma no invasiva, rápida y precisa las distintas anormalidades, otorgando de esta manera información sumamente útil a los cirujanos cardiovasculares para definir la estrategia quirúrgica. En el presente artículo revisamos las aplicaciones de la angiotomografia computada multicorte en la evaluación de las malformaciones congénitas de la arteria aorta más frecuentes en el periodo neonatal y en la primera infancia Palabras clave: Arteria aorta. Anomalías congénitas. Neonatos. Angiotomografia computada multicorte. RAR Resumen 2009 Diego Haberman1, Enrique Gurfinkel1, Alejandro Beresñak1, Adriana Martínez1, Rosa Emsani1, Rubén Toledo2 Congenital abnormalities of aortic artery Número 3 2009 Table 1 The heart begins a process of folding and septation. Subsequently, the cardiac bulb divides in three parts: proximal, one intermediate called truncus arteriosus and distal, called aortic sac. The two big arteries that are born in the heart are not originated from preexisting vessels, but from the truncus arteriosus, which first forms a partition and then divides to form two independent vessels: the ascending aortic artery and the pulmonary artery trunk. From the aortic sac there 6 pairs of aortic arches connecting the ventral and dorsal aortic sketches are born. Some of them usually regress with development, persisting at the end of the fifth week only third, fourth and sixth arcs, which will later originate the great vessels (1). Patient Gender Age Diagnosis DV M 5 months Incomplete aortic ring LI M 3 weeks Truncus arteriosus type II MM F 2 weeks Coarctation PM F 11 months Aberrant subclavian right artery PS F 6 days Interruption of aortic arch type B SA F 2 months Coarctation RM F 11 months Coarctation VG M 7 days Coarctation LC F 5 months Interruption of aortic arch type A RESULTS PM F 6 months Interruption of aortic arch type A SN M 1 month Coarctation PA M 1 month Truncus arteriosus type III In the present study, twelve symptomatic patients were included, all of them with a previous echocardiography. One patient with coarctation diagnosis by echocardiography, was interpreted by CTA as an interruption of aortic arch type A, which was confirmed by surgery. In the other 11 patients, there was a coincidence between the echo-cardiography and the CTA. None of our patients underwent MRI. SN M 1 mes Coartación PA M 1 mes Tronco arterioso tipo III Coarctation This is the most common entity in our series, with 5 patients studied with preoperative CTA (41.6%). Fig. 1. Male patient, with 6 days of life. 3D reconstruction. Sagital plane. Coarctation of typical localization in descending aorta, distal to the appearance of left subclavian artery (arrow). Relative hipoplasia of transverse arch portion (arrowhead). Fig. 2. Female patient, with 14 days of life. Signs of heart failure, pulse discrepancies and dorsal systolic murmur. Sagital plane. Maximum intensity reconstruction. Severe coarctation (arrow) with continuity to descending aorta through a filiform flow and intercostals collateral circulation (arrowhead). A.M.I: Internal mammal artery. In surgery, the diagnosis is proved. Resection was performed for coarctation with end to end anastomosis and ligation of the ductus, which was permeable (not visible in ACT). Página 2 RAR Volumen 73 Clinical cases of two cardiac tubes, arterial and venous vessels. Subsequently, both cardiac tubes fuse to form a tubular heart that develops four dilatations named cavernous sinus, atrium, venticulum and cardiac bulb. Diego Haberman et al. a b b Volumen 73 Número 3 a 2009 Fig. 3. a) Female patient, 2 months old. Coronal plane: Situs inversus totalis plus coarctation (arrow). A.S.D.: Right subclavian artery. A.I: Left atrium. A right torocotomia is performed, coarctation section and end to end anastomosis . b) 3D reconstruction. Posterior PLANE. Coarctation (arrow). A.S.D: Right subclavian artery. T: trachea. Scheme 1: Illustrative scheme of the interruption of aortic arch type A. VD: Right ventricle. VI: Left ventricle. AP: Pulmonary artery. A Ao Asc: Ascending aortic artery. A Ao Desc: Descending aortic artery. Tr Bc: Brachiocephalic arterial trunk. ACPI: Left primitive carotid artery. ASI: Left subclavian artery. Other cardiovascular abnormalities are usually presented, among them, bicuspid aortic valve, hipoplastic transverse arch portion, dilatation of the ascending aorta, persistent ductus and aberrant right subclavian artery (4) (Fig.1). There is hypertension of superior extremity, which Página 3 It is a congenital abnormality characterized by narrowing of the aortic lumen. Stenosis is often found in the proximal descending aorta after the emergence of left subclavian artery. Considering its connection to the ductus arteriosus, they are classified in preductal, postductal or yuxtaductal (2, 3). RAR c Fig. 4. a) Female patient. Interruption of aortic arch type A. Sagital plane. Maximum intensity reconstruction. Thoracic artery is interrupted in the posterior sector of the arch (arrow), in distal position to the left subclavian artery (ASI). The descending aorta receives flow from the pulmonary aorta (AP) through a permeable ductus (arrowhead). b) 3D reconstruction. Anterior oblique left plane. Ao. A.: Ascending aorta. Ao. D.: Descending aorta. AP: Pulmonary artery. ASI: Left subclavian artery. Interruption of aortic arch (arrow). c) 3D reconstruction. Posterior oblique plane. APD: Right pulmonary artery. API: Left pulmonary artery. ACPI: Left primitive carotid artery. ASI: Left subclavian artery. Ao. D.: Descending aorta. In the surgery, the ductus is joint. En la cirugía se liga el ductus, se reseca la zona interrumpida con posterior anastomosis que involucra el arco aórtico, la boca de la arteria subclavia y la aorta descendente. Congenital abnormalities of aortic artery b c d c Fig. 5. a) Female patient, with 5 days of life. Respiratory distress. Cardiogenic shock. Aortic interruption type B. 3D reconstruction. There is a dissociation between ascending and descending aorta, which has a continuity with the trunk of pulmonary artery. The interruption is between carotid artery and left subclavian, which emerges from the descending aorta. Ao. A.: Ascending aorta. A.C.P.I: Left primitive carotid artery. ASI: Left subclavian artery. b) 3D reconstruction. Posterior plane. Ao. A.: Ascending aorta. Ao. D.: Descending aorta. A.P.D: Right pulmonary artery. ASI: Left subclavian artery. c) Sagital plane. The pulmonary artery (AP), dilated, continues with the descending aorta (Ao. D), and from it, the left subclavian artery (ASI) is originated. d and e) Coronal plane. The aorta and pulmonary artery arise from one of the corresponding ventricles. V.I.: Left ventricle. V.D.: Right ventricle. A.P.: Pulmonary artery. Ao.A.: Ascending aorta. RAR Volumen 73 Número 3 2009 a Página 4 tends to be offset by collateral development of intercostals, epigastrics and mediastinals (Fig. 2). Infants with coarctation, particularly when a persisting ductus arteriosus exists, presented with signs of congestive heart failure (tachycardia and dyspnea). A dorsal systolic murmur is often listened, pulses are verified and differential pressures in the lower limbs. Diagnosis is based on the clinical findings and on imaging methods (echocardiography, angiography, angiotomography and magnetic resonance). The CT scan gives us information about the site and extension of stenosis, aspect of collaterals and the presence of associated abnormalities, currently an extremely useful modality in the following of postpercutaneous or surgery treatment (Fig 3). Scheme 2: Illustrative scheme of the disruption of aortic arch type B. VD: Right ventricle. VI: Left ventricle. AP: Pulmonary artery. A Ao Asc: Ascending aortic artery. A Ao Desc: Descending aortic artery. Tr Bc: Brachiocephalic arterial trunk. ACPI: Left primitive carotid artery. ASI: Left subclavian artery. Interruption of aortic arch It is defined as the loss of anatomic continuity between the ascending and the descending aorta, and it is considered as an extreme form of coarctation. The flow towards the descending aorta is given by the ductus, which remains permeable and provides continuity between the trunk of the pulmonary artery and the distal aorta to the interruption. Despite this continuity, the blood that arrives to the abdomen and lower limbs is only partially oxygenated. In most of the cases, other cardiovascular abnormalities are associated, such as interventricle communication (IVC), obstruction in the exit tract of left ventricle, bicuspid aortic valve and persisting arterial ductus. Patients evolve fast after birth with severe congestive heart failure, shock tendency and, if not treated, they often pass away in the neonatal period at few days of birth (5). Diego Haberman et al. Truncus arteriosus The truncus arteriosus (TA) is characterized by the presence of a single large artery which originates in the base of the heart with a single annulus, that originates coronary, pulmonary and systemic arteries. In most of the cases there is an interventricular communication and the trunk usually originates over this septal defect. It is the result of a failure in the early embryonic development due to a lack of division of the primary arterial precursor (truncus arteriosus) in aortic and pulmonary arteries, eventuality that must happen in the normal fetus at approximately 8 weeks of gestation. It is a pathology with low incidence, and represents approximately the 0.7% of cardiovascular congenital defects. It can be associated to other alterations, among them, the aortic arch to the right, coarctation and interruption of the aortic arch (association which is present in the 14% of TA), and also to extracardiac abnormalities, particularly Di George syndro- Número 3 Volumen 73 Vascular rings They belong to abnormal configurations of aortic arch, which are the result of an incomplete regression of one of the six present bronchial arches during the embryonic development. Vascular structures or arterial ligament surround and compress the trachea and esophagus, generating symptoms such as stridor, breathing difficulty, air trapping and apnea; the esophagus compression causes vomits, swallowing difficulty and dysphagia. They predispose to the appearance of aspiration pneumonias (12). They can be complete, forming a continuous ring around the trachea and the esophagus, or incomplete, in such cases the symptoms are usually minor or even absent (13). There are several forms of vascular rings: Syndrome of the anomalous innominate artery. The brachiocephalic arterial trunk originates from the aortic arch but more to the left than usual, being the reason for which it crosses diagonally ahead the trachea and can have certain degree of compression on its anterior side. Double aortic arch. It can exist as complete aortic arch (functional), in which there is persistence and permeability of right and left arch, or as double arch with partial atresia of one of them (usually, the left one), where the continuity is given by a remaining RAR Its incidence is low, 3 cases at a million of born alive approximately, and represents the 1% of all cardiovascular abnormalities. In our study, there were 3 of the 12 studied patients. They have been classified in three types according to the place where the interruption is,: Type A: Distal to the left subclavian artery. Type B: Between the common left carotid artery and the left subclavian artery. Type C: Between the brachiocephalic arterial trunk and the left subclavian artery. Type B is the most frequent one (53%), followed by type A (43%) and type C (4%) (6). The ACT clearly shows the interruption, its connection to the supra-aortic trunks, as well as the presence of the ductus, that provides continuity between the pulmonary artery and the descendent aorta (Schemes 1 and 2) (Fig. 4, 5, 6). Página 5 Figure 6. Surgical photography. A.P.: dilated pulmonary artery. Ao.A.: Ascending aorta. Collet-Edwards classification Type I. There is a small trunk that is separated. Right and left branches of the pulmonary artery are originated from it. Type II. Both arteries are born from the posterior side of TA, separated by a short distance. Type III. Each artery is born independently of the TA’s lateral side, one away from the other. Type IV. One branch is born from the trunk and the other, from the descendent aorta or the ductus. Van Praagh questions Collet’s classification, specially type IV, and introduces some modifications considering the presence or absence of interventricular communication and interruption of aortic arch. He uses the prefix A to indicate the presence of IVC, or B to indicate the intact septum. On the other hand, he includes in type IV the TA associated to the interruption of the arch (10). There is a pulmonary flow increased with a mixture of systemic and pulmonary blood. Most of the children arrive with cyanosis in early stages due to the mixture of oxygenated and deoxidized blood and with signs of congestive heart failure. The prognosis, if a surgery correction is not performed, is bad, with a survival of 25% at 6 months (11) (Schemes 3 and 4) (Fig. 7, 8). 2009 me (facial anomalies, cleft palate hypoplastic thymus and hipocalcemia) (7). There are two anatomic classification systems that basically consider the origin of pulmonary arteries: Collet-Edwards and Van Praagh (8, 9). Congenital abnormalities of aortic artery a b c d Página 6 RAR Volumen 73 Número 3 2009 Scheme 3: Illustrative scheme of truncus arteriosus type III. VD: Right ventricle. VI: Left ventricle. CIV: Ventricular septal defect. TA: Truncus arteriosus. APD: Right pulmonary artery. API: Left pulmonary artery. Figure 7. a) Male patient, with 3 weeks of life. Dyspnea, tachycardia and cyanosis. Truncus arteriosus type III. Coronal plane. Extensive ventricular septal defect (C.I.V) above which originates a single large artery called truncus arteriosus (TA). V.I.: Left ventricle. V.D.: Right ventricle. A.D.: Right atrium. b) Coronal plane. Picture at 5 mm posterior to previous photo. From the truncus arteriosus (TA) is fromed a small common trunk that will form the brachiocephalic arterial trunk (Tr.B.) and left primitive carotid artery (A.C.P.I.). c) The right pulmonary artery originates from the lateral side of the truncus arteriosus (T.A.). d) Plane cut at 5 mm posterior to the previous image. Left lung artery (API) is born far away from and at different heights of the right pulmonary artery. At the same level, the truncus is continuous with the descending aorta. fibrous cord that completes the ring. This last option is the most frequent one. Supra-aortic trunks appear separately, without a brachiocephalic arterial trunk, this is, subclavia artery and right carotid, subclavia artery and left carotid, which is named sign of the four vessels. When the minor arch is atresic, the fibrous cord generally appears distal to the appearance of left subclavian artery. In these cases, it can sometimes be misinterpreted as an aortic arch to the right; however, the sign of the four vessels is usually seen. On the other hand, on the atresic side, a small permeable segment previous to the fibrous cord usually remains, manifested as a diverticulum posterior to the esophagus (Scheme 5) (Fig. 9). Aortic arch to the left with aberrant right subclavian artery. In the literature it is not considered as a true ring. However, in some occasions, it causes considerable symptoms, similarly to true rings. In these patients, the right subclavian artery is not originated from the brachiocephalic arterial trunk, since it branches off as the last vessel of the aortic arch and courses posterior to the esophagus upwards to the right (Fig. 10). Aortic arch to the right with aberrant left subclavian artery. The aberrant left subclavian artery is the last trunk to branch off. The presence of the arterial ligament completes a true ring. They usually are symptomatic at early age. DISCUSSION Traditionally, congenital abnormalities of aortic arch were evaluated with echo-cardiography or conventional radiology and, in some instances, with conventional angiography. In recent years, magnetic resonance (MR) and ACT, specially with multislice technology, gained relevance in the diagnosis and pre-surgery scan of different abnormalities (14-16). Each one of these modalities presents advantages and disadvantages that must be considered in choosing one. Neonate patients and little infants present a heart rate that oscillates between 100 and 160 heart beats a minute. Therefore, it is essential to count with equip- Diego Haberman et al. a b c d ment of high temporal resolution in order to reduce the movements per heart beat, decreasing also the time exposure to radiation and the anesthetic time, and also with high space resolution due to the small size of the organs to study. Multislice computer tomography achieves these specifications. In 64 detector units the space resolution is of 0.35mm and the temporal resolution is of 0.4 seconds, enabling to obtain, in an apnea of 3 to 5 seconds, an acquisition of the complete thorax of a neonate or a little infant. It only takes one volumetric acquisition to subsequently do multiplanar and three dimensional reconstructions. With ACT it is possible to perform “triggered” or synchronized exams, obtaining images in different phases of the EKG, which allows to reconstruct them in the lowest movement phase and to reduce the artifacts per beat. However, triggered acquisition adds more radiation, for what it is used in a few occasions, when it is absolutely necessary to define the aortic root’s anatomy (where there usually is more movement). The disadvantage of ACT is the utilization of radiation and eventual adverse reactions to iodine. With high field MR units and fast sequences, images of high quality and millimeter space resolution are obtained. Planar sequences are obtained in different orientations, and also images at up 20 phases during the cardiac cycle that are visualized as film and 3D angiographic sequences (17). One of the advantages of this method is that it provides morphological and functional information, with the possibility to measure flow and pressure gradients. This can be used as a predictor of severity in situations such as coarctation (18). In addition, it enables to visualize the anatomy and function of heart valves, which is useful in the aortic arch anomalies that are associated to valve malformations (coarctation- bicuspid aortic valve). Another advantage of MR is that it does not use ionizating radiation, which is important when control repetition studies are needed. One disadvantage is the acquisition time, which can be a limitation for the study of patients with bad general condition who cannot tolerate prolonged Número 3 Volumen 73 RAR Figure 8. Truncus arteriosus type II. 3D reconstruction. Previous view. From the truncus arteriosus (T.A.) emerges a small common trunk (T.C.) that will give rise to the left primitive carotid artery (ACPI) and the brachiocephalic arterial trunk (Tr.Br.). Truncus arteriosus continues with the descending aorta (Ao. D.) through permeable ductus (Du). A.C.P.D.: right primitive carotid artery. A.S.D.: right subclavian artery. A.S.I.: left subclavian artery. b) 3D reconstruction. Anterior oblique view. T.A.: truncus arteriosus. Du.: Ductus. Ao.D.: Descending aorta. A.S.I.: left subclavian artery. c) 3D reconstruction. Posterior view. Lung arteries originate close together from the back of the truncus arteriosus. A.P.D.: Right pulmonary artery. A.P.I.: left pulmonary artery. Ao.D.: Descending aorta. d) Surgical photography. T.A.: truncus arteriosus. T.C.: common trunk. V.C.S.: superior vena cava. Página 7 VD: Right ventricle. VI: Left ventricle. CIV: Ventricular septal defect. TA: Truncus arteriosus. APD: Right pulmonary artery. API: Left pulmonary artery. 2009 Scheme 4: Illustrative scheme of truncus arteriosus type II. Congenital abnormalities of aortic artery a b Número 3 2009 c Figure 9. a) Male patient, 5 months old. Progressive respiratory distress and stridor. Double aortic arch with atresia of the left component. Coronal plane. Diverticulum of the left atretic arch (arrow). A.S.D.: right subclavian artery. A.S.I.: left subclavian artery. b) 3D reconstruction. Superior oblique view. Emerging arteries from the aortic arch emerge each independently (Sign of the four vessels). A.C.P.I.: Left primitive carotid artery. A.C.P.D.: right primitive carotid artery. A.S.I.: left subclavian artery. A.S.D.: right subclavian artery. Diverticulum of the left atretic arch (arrow). c) Axial plane. Compression of right main bronchus with signs of air trapping of that lung. b c RAR Volumen 73 a Scheme 5: Illustrative scheme of incomplete double aortic arch. Diverticulum corresponding to the permeable sector of the double arch (long arrow). Fibrous cord that completes the arch (arrowhead). Figure 10. a) Female patient, 11 months old. Dysphagia and recurrent pneumonias. Axial plane. Aberrant right subclavian artery with retroesophageal path. Airspace consolidations on the right upper lobe with some hypodense sectors with aspiration pneumonia look. A.S.D.: right subclavian artery. T: Trachea. Dilated esophagus (arrow). b) A.S.D.: right subclavian artery. Ao.D.: Descending aorta. c) 3D reconstruction. Posterior view. A.S.D.: right subclavian artery. A.S.I.: left subclavian artery. A.C.P.D.: right primitive carotid artery. A.C.P.I.: left primitive carotid artery. With surgery, it is performed a right posterolateral thoracotomy, section of the aberrant artery at the base and its reimplantation in the ipsilateral carotid artery. Página 8 anesthesia. Studies take between 20 – 40 minutes, longer than ACT. The decision of which modality is to be used, depends on the availability and experience of each particular imaging service. In our opinion, CT presents the advantage of being extremely fast and easy to program, requiring less complex anesthetic procedures. CONCLUSION Computed tomography angiography appears as an extremely useful complementary method in the diagnosis of congenital abnormalities of the aortic arch in neonates and early childhood patients. It is a fast and non invasive procedure, which takes short anesthetic time and low charge of intravenous contrast. In our series, it enabled to diagnose all the studied anomalies in an efficient and precise way, with excellent correlation to the surgery findings reported by surgeons. 1. 11. 2. 3. 4. 5. 6. 7. 8. Hib, José. Embriología médica. 7ª Ed. Santiago- Chile: McGraw-Hill / Interamericana de Chile; 1999. p. 124-141. Becker C, Soppa C, Fink U, et al. Spiral CT angiography and 3D reconstruction in patients with aortic coarctation. Eur Radiol 1997;7(9):1473-1477. Lee EY, Siegel MJ, Hildebolt CF, Gutierrez FR, Bhalla S, Fallah JH. MDCT Evaluation of thoracic aortic anomalies in pediatric patients and young adults: comparison of axial, multiplanar, and 3d images. AJR Am J Roentgenol 2004;182:777-784 Sebastià C, Quiroga S, Boyé R, Perez-Lafuente M, Castellà E, Alvarez-Castells A. Aortic stenosis: spectrum of diseases depicted at multisection CT. RadioGraphics 2003;23;S79–91 Collins-Nakai RL, Dick M, Parisi-Buckley L, Fyler DC, Castaneda AR. Interrupted aortic arch in infancy. J Pediatric 1976;88:958-962. Celoria GC, Patton RB. Congenital absence of the aortic arch. Am Heart J 1959;58:407-413. Konstantinov IE, Karamlou T, Blackstone EH, et al. Truncus arteriosus associated with interrupted aortic arch in 50 neonates: a Congenital Heart Surgeons Society Study. Ann Thorac Surg 2006;81;214-223. Collet RW, Edwards JE. Persistent Truncus arteriosus: A classification according anatomic types. Surg Clin North Am 1949;29(4):1245-70. 12. 13. 14. 15. 16. 17. 18. Número 3 10. Volumen 73 Bibliography Van Praagh, S; Van Praagh R. The anatomy of common aortopulmonary trunk (truncus arteriosus communis) and its embryologic implications. A study of 57 necropsy cases. Am J Cardiol 1965;16:406-25. Van Praagh R. Truncus arteriosus: what is it really and how should it be classified. Eur J Cardiothorac Surgery 1987;1(2):65:70. Caffey. Diagnóstico por imágenes en pediatría. Buenos Aires: Editorial Médica Panamericana; 1992. p. 424-425. Lowe G, Donaldson J, Backer C. Vascular Rings: 10 years review of imaging. Radiographics 1991;11:637-646. Berrocal T, Madrid C, Novo S, Gutiérrez J, Arjonilla A, Gómez-León N. Congenital anomalies of the tracheobronchial tree, lung and mediastinum: embryology, radiology and pathology. Radiographics 2004;24(1):e-17. Haramati LB, Glickstein JS, Issenberg HJ, Haramati N, Crooke GA. MR Imaging and CT of vascular anomalies and connections in patients with congenital heart disease: significance in surgical planning. Radiographics 2002;22:337-349. Kersting-Sommerhoff BA, Sechtem UP, Fisher MR, Higgins CB. MR imaging of congenital anomalies of the aortic arch. AJR Am J Roentgenol 1987;149:9-13,. Goo HW, Park IS, Ko JK, et al. CT of congenital heart disease: normal anatomy and typical pathologic conditions. RadioGraphics 2003;23:S147–S165 Kellenberger CJ, Yoo SJ, Büchel ER. Cardiovascular MR imaging in neonates and infants with congenital heart disease. RadioGraphics 2007;27:5–18. Nielsen JC, Powell AJ, Gauvreau K, Marcus EN, Prakash A, Geva T. Magnetic Resonance imaging predictors of coarctation severity. Circulation. 2005;111:622-628. RAR 9. Página 9 Acknowledgements: Radiographers Gustavo Bisignano, Adrián Mancinelli, Héctor Juárez, Alejandro Steimberg. 2009 Diego Haberman et al.