* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The Digestive system
Survey
Document related concepts
Transcript
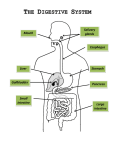
كلية الصيدلة والعلوم الطبية قسم التغذية « من قيمنا » • تثمين اإلبداع واإلنجاز Eat breakfast like a king, lunch like a prince, and dinner like a beggar. The Digestive system Chapter 18 (Pages 620-654) Unlike plants, which can form organic molecules using inorganic compounds such as carbon dioxide, water, and ammonia, humans and other animals must obtain their basic organic molecules from food. Some of the ingested food molecules are needed for: 1. Their energy value (obtained by reactions of cell respiration and used in the production of ATP), 2. To make additional tissues. Most of the organic molecules that are ingested are similar to the molecules that form human tissues. These are generally large molecules (polymers), which are composed of subunits (monomers). Review: Polymers/Monomers. Hydrolysis reactions. (Fig. 18.1 page 620) Within the gastrointestinal tract, the digestion of these large molecules into their monomers occurs by means of hydrolysis reactions. The monomers thus formed are transported across the wall of the small intestine into the blood and lymph in the process of absorption. Digestion and absorption are the primary functions of the digestive system. Fig. 18.1 The digestion of food molecules through hydrolysis reactions. These reactions ultimately release the subunits molecules of each food category. Because the composition of food is similar to the composition of the body tissues, enzymes that digest food are also capable of digesting a persons own tissues. This does not normally occur because a variety of protective devices inactivate digestive enzymes in the body and keep them away from the cytoplasm of the cells. The fully active digestive enzymes are normally confined the lumen (cavity) of the gastrointestinal tract. The lumen of the gastrointestinal tract is open at both ends (mouth and anus), and is thus continuous with the environment. The harsh conditions required for digestion occur outside the body. Indigestible materials, such as cellulose from plant walls, pass from one end to the other without crossing the epithelial lining of the digestive tract; since they are not absorbed, they do not enter the body. The 2 open ends of the digestive tract permit one-way transport, which is ensured by wavelike muscle contractions and by the action of sphincter muscles. This one-way transport allows different regions of the gastrointestinal tract to be specialized for different functions. Fig. 18.2 The organs of the digestive system. The digestive system includes the gastrointestinal tract and the accessory digestive organs. Functions of the digestive system: 1.Motility: movement of food through the digestive tract through the processes of : a. Ingestion: taking the food into the mouth. b. Mastication: chewing and mixing food with saliva. c. Deglutition: swallowing the food. d. Peristalsis: rhythmic, wave-like contractions (peristalsis), and mixing contractions in different segments (segmentation), move food through the gastrointestinal (GI) tract. 2.Secretion: include: a. Exocrine secretions: H2O, HCl, HCO3- , and many digestive enzymes are secreted into the lumen of GI tract. b. Endocrine secretions: Stomach and small intestine secrete a number of hormones that help to regulate the digestive system. 3.Digestion: breakdown of food into small subunits. 4.Absorption: passage of digested food into blood or lymph. 5.Storage and Elimination: temporary storage and subsequent elimination of indigestible food molecules. 6. Immune barrier: The simple columnar epithelium that lines the intestine, with its junctions between cells, provides a physical barrier to the penetration of pathological organisms that their toxins. Also, cells of the immune system reside in the connective tissue located just under the epithelium to promote immune responses. Anatomically and functionally, the digestive system can be divided into: (Fig. 18.2 page 621) 1. Gastrointestinal (GI) tract or alimentary canal: Is about 9 meters (30 feet) long. Extend from the mouth to the anus. Traverses the thoracic cavity and enters the abdominal cavity at the level of the diaphragm. Anus is located at the inferior portion of the pelvic cavity. Organs of the GI tract are: Oral cavity, Pharynx, Esophagus, Stomach, Small and Large intestine. 2. Accessory digestive organs: include: Teeth, Tongue, Salivary gland, Liver, Gall bladder, and Pancreas. [Viscera is frequently used to refer to the abdominal organs of digestion, but it also can be used in reference to any organs in the thoracic and abdominal cavities] Layers of the GI tract (Fig. 18.3 page 622) From the esophagus to the anal canal, the GI tract is composed of layers or tunics. Each tunic contains a dominant tissue type that performs specific functions in the digestive process. The 4 tunics are: (from inside to outside) 1. Mucosa: Lines the lumen of the GI tract. Is the absorptive and major secretory layer. Consists of simple columnar epithelium supported by lamina propria which is a thin layer of areolar connective tissue containing numerous lymph nodules which are important in protecting against disease. External to lamina propria is a thin layer of smooth muscle called muscularis mucosae. This is the muscle layer responsible for the numerous small folds in certain portions of the GI tract. These folds greatly increase the absorptive surface area. Specialized goblet cells in the mucosa secrete mucus throughout most of the GI tract. 2. Submucosa: Relatively thick connective tissue. Highly vascular layer that serves the mucosa. Absorbed molecules that pass through the columnar epithelial cells of the mucosa enter into blood and lymphatic vessels of the submucosa. Contains glands and nerve plexuses called Meissner's plexus that provides a nerve supply to the muscularis mucosae of the small and large intestine. 3. Muscularis: Also called muscularis externa. Responsible for segmental contractions and peristaltic movement through the GI tract. Has inner circular and outer longitudinal layer of smooth muscle. Its contractions move the food through the tract and physically pulverize and mix the food with the digestive enzymes. Myenteric plexus (Auerbach's plexus) located between two muscle layers, provides the major nerve supply to the entire GI tract. Myenteric plexus includes fibers and ganglia from both the sympathetic and parasympathetic divisions of the autonomic nervous system (A.N.S.). 4. Serosa: Outer layer that completes the wall of the GI tract. Consist of areolar connective tissue covered with a layer of simple squamous epithelium. Function in binding and protection. Fig. 18.3 The layers of the digestive tract. (a) An illustration of the major tunics, or layers, of the small intestine. The insert shows how folds of mucosa form projections called villi in the small intestine. (b) An illustration of a cross section of the small intestine showing layers and glands. الخبرة هي عدد األخطاء التي ارتكبتها في حياتك السابقة From Mouth to Stomach Mouth 1.Mastication (chewing) of food mixes it with saliva, secreted by salivary glands. In addition to mucus, and various antimicrobial agent, saliva contains salivary amylase, an enzyme that can catalyze the partial digestion of starch. 2.Deglutition (swallowing) is divided into 3 phases: a. Oral. b. Pharyngeal. c. Esophageal. The oral phase is under voluntary control, while the pharyngeal and esophageal phases are automatic and controlled by the swallowing center in the brain stem. In the oral phase, the muscles of the mouth and tongue mix the food with saliva and create a bolus (a mass of a size to be swallowed) of food that the tongue muscles move toward the oropharynx. Esophagus (Fig. 18.4 page 624) Connects pharynx to the stomach. Muscular tube about 25 cm (10 in) in length. Located posterior to the trachea within the mediastinum of the thorax. Swallowed food is pushed from the oral to the anal end of the esophagus (and, afterward, of the intestine) by a wavelike muscular contraction called peristalsis. Movement of the bolus along the digestive tract occurs because the circular smooth muscles contracts behind, and relaxes in front of, the bolus. Lumen of the terminal portion of esophagus is slightly narrowed because of the circular muscle fibers in its wall. This portion is called the lower esophageal (gastroesophageal) sphincter. After food passes into the stomach, constriction of this sphincter helps to prevent the stomach content from regurgitating into the esophagus. Regurgitation would occur because the pressure in the abdominal cavity is greater than the pressure in the thoracic cavity as a result of respiratory movements. The lower esophageal sphincter must therefore remain closed until food is pushed through it by peristalsis into the stomach. Figure 18.4 Peristalsis in the esophagus Stomach (Fig. 18.5 page 624) Is a J-shaped Is the most distensible part of the GI tract. Superiorly is continuous with the esophagus, and inferiorly empties into the duodenum. Functions: 1. Storage of food. 2. Initiation of proteins digestion. 3. Kill bacteria with the strong acidity of gastric juice. 4. Move the food (chyme) into the small intestine. Regions: 1. Cardiac region: composed of: a. Upper fundus b. Lower body. The 2 together compose about 2/3rds of the stomach. 2. Pyloric region: is the distal portion of the stomach. Composed of: a. Antrum. b. Pyloric sphincter. Figure 18.5 Primary regions and structures of the stomach. Notice that the pyloric region of the stomach includes the pyloric antrum as well as the pyloric sphincter. Contractions of the stomach: 1. Churn the chyme; mixing it more thoroughly with the gastric secretions. 2. Push partially digested food from the antrum through the pyloric sphincter into the duodenum. The inner surface of stomach has long folds called rugae. Gastric mucosa is folded which open into the stomach lumen, and the openings are called gastric pits. Cells that line the folds deeper in the mucosa secrete various products into the stomach; these cells form the exocrine gastric glands. Gastric glands contain several types of cells that secrete different products: (Fig.18.6 page 625) 1. Mucus neck cells, which secrete mucus. 2.Parietal cells: secrete HCl and probably the intrinsic factor which is required for the intestinal absorption of vitamin B12. 3.Chief (Zymogenic) cells: secrete pepsinogen (an inactive form of the proteindigesting enzyme pepsin). 4.Enterochromaffin-like (ECL) cells: found in the stomach and intestine, which secrete histamine and 5-hydroxytryptamine (serotonin) as paracrine regulators of the GI tract. 5.G cells: secrete the hormone gastrin into the blood. 6.D cells: secrete the hormone somatostatin. Fig. 18.6 Gastric pits and gastric glands of the mucosa. (a) Gastric pits are openings of the gastric glands. (b) Gastric glands consist of several types of cells (including mucous cells, chief cells, and parietal cells), each of which produces a specific secretion. Gastric pits and gastric glands of the mucosa Stomach secretes a hormone named ghrelin which rises before meals and falls after meals. Ghrelin may serve as a signal from the stomach to the brain that helps regulate hunger. The exocrine secretions of the gastric cells, together with a large amount of H2O (2-4L/day) form a highly acidic solution known as gastric juice. Pepsin and HCl secretion The parietal cells secrete H+, at a pH as low as 0.8, into the gastric lumen by primary active transport (involving carriers that function as an ATPase) (Fig. 18.8 page 608). These carriers, known as H+/K+ ATPase pumps transport H+ uphill aginst a millionto-one concentration gradient into the lumen of the stomach while they transport K+ in the opposite direction. At the same time, the parietal cell's basolateral membrane takes in Cl- against its electrochemical gradient by coupling its transport to the downhill movement of HCO3-. HCO3- is produced within the parietal cell by the dissociation of H2CO3, formed from CO2 and H2O by the enzyme carbonic anhydrase. Therefore, the parietal cell can secrete Cl- (by facilitative diffusion) as well as H+ into the gastric juice while it secretes HCO3- into the blood. Figure 18.7 Secretion of gastric acid by parietal cells. The apical membrane (facing the lumen) secretes (H+) in exchange for (K+) using a primary active transport that is powered by the hydrolysis of ATP. The basolateral membrane (facing the blood) secretes (HCO3-) in exchange for Cl-. The Cl- moves into the cell against it electrochemical gradient, powered by the downhill movement of (HCO3-) out of the cell. This (HCO3-) is produced by the dissociation of H2CO3,which is formed from CO2 and H2O by the enzyme carbonic anhydrase. The Cl- then leaves the apical portion of the membrane by diffusion through a membrane channel. The parietal cells thus secrete HCl into the stomach lumen as they secrete (HCO3-) into the blood. The secretion of HCl by the parietal cells is stimulated by gastrin, histamine, and particular neurotransmitters. Gastrin, secreted by G cells, is carried in the general circulation to the parietal cells, where it stimulates acid secretion. Also it stimulates ECL cells to secrete histamine, which acts as a paracrine regulator to stimulate HCl secretion by parietal cells (Fig. 18.31 page 622). The high concentration of HCl makes gastric juice very acidic, with a pH of < 2. The strong acidity serves 3 functions: 1.Denaturation of proteins, thus becoming more digestible. 2.Activation of pepsinogen (Fig.18.9 page 605) 3.Pepsin is more active under acidic conditions. Figure 18.30 The regulation of gastric acid secretion. The presence of amino acids in the stomach lumen from partially digested proteins stimulates gastrin secretion. Gastrin secretion from G cells is also stimulated by vagus nerve activity. The secreted gastrin then acts as a hormone to stimulate histamine release from ECL cells. The histamine, in turn, acts as a paracrine regulator to stimulate the parietal cells to secrete HCl. ( + stimulation; Θ inhibition.) Figure 18.8 The activation of pepsin. The gastric mucosa secretes the inactive enzyme pepsinogen and hydrochloric acid (HCl). In the presence of HCl, the active enzyme pepsin is produced. Pepsin digests protein into shorter polypeptides. As a result of the activation of pepsin under acidic conditions, the fully active pepsin is able to catalyze the hydrolysis of peptide bonds in the ingested protein. Thus, the cooperative activities of pepsin and HCl permit the partial digestion of food protein in the stomach. The strong acid and protein-digesting action of pepsin could damage the lining of the stomach producing peptic ulcer. The stomach defends itself against damage by: 1.Presence of a stable gel of mucus called adherent layer of mucus that is adherent (stuck) to the gastric epithelial surface. The layer contains alkaline bicarbonate, secreted from the apical plasma membranes of the epithelial cells. When the stomach secretes more acid into the lumen, there is also more bicarbonate available to the epithelial cell for secretion into the mucus. As a result, the pH at the epithelial surface is normally near neutral. The adherent layer of gastric mucus is also the major barrier to potential damage to the stomach caused by pepsin. 2. Presence of tight junctions between adjacent epithelial cells, preventing acid and pepsin from leaking past the epithelial barrier and destroying underlying tissues. 3. A rapid rate of epithelial cell division replaces the entire epithelium every 3 days, so that the damaged cells can rapidly be replaced. We Plan big things for tomorrow in spite of zero knowledge of the future. That’s CONFIDEMCE Digestion and Absorption in the Stomach Proteins are only partially digested in the stomach by the action of pepsin. Carbohydrates and fats are not digested at all by pepsin. (The salivary amylase soon becomes inactivated by the strong acidity of gastric juice.) The complete digestion of food molecules occurs later, when chyme enters the small intestine. Therefore, people who had partial gastric resections (and even those who have had complete gastrectomies) can still adequately digest and absorb their food. Almost all of the products of digestion are absorbed through the wall of the small intestine. The only commonly ingested substances that can be absorbed across the stomach wall are alcohol and aspirin. (Passage of aspirin through the gastric mucosa has been shown to cause bleeding.) Absorption occurs as a result of the lipid solubility of these molecules. Gastritis and Peptic Ulcers Peptic ulcers are erosions of the mucous membranes of the stomach or duodenum produced by the action of HCl that penetrate through the muscularis mucosa layer. Excessive gastric acid secretion can cause ulcers of the duodenum. Ulcers of the stomach are not believed to be due to excessive acid secretion, but rather to mechanisms that reduce the barriers of the gastric mucosa to selfdigestion. Most people who have peptic ulcers are infected with Helicobacter pylori, which have adaptations that enable them to survive in the very acidic pH of the stomach and infect almost half the adult population worldwide. Scientists discovered that this common bacterium, rather that emotional stress or spicy food, is the cause of most cases of peptic ulcers of the stomach and duodenum. As a result of this discovery, ulcers can now be effectively treated medically. When the gastric barriers to self-digestion are broken down: 1.Acid can leak through the mucosa to the submucosa, causing direct damage and stimulating inflammation. 2.Histamine released from mast cells during inflammation may stimulate further acids secretion and result in further damage to the mucosa. 3.The inflammation that occurs during these events is called acute gastritis. The duodenum is normally protected from gastric acid by: 1.The adherent layer of mucus on its epithelium. Duodenal cells secrete bicarbonate into this adherent mucus layer, so that the surface epithelium is normally exposed to a neutral pH. 2.Secretion of bicarbonate by Brunner's glands in the submucosa of the duodenum. 3. Acidic chyme is neutralized by the buffering action of bicarbonate in alkaline pancreatic juice, which is released into the duodenum upon the arrival of the acidic chyme from the stomach. Duodenal ulcers may result from excessive gastric acid secretions and/or inadequate secretion of bicarbonate in the duodenum. People with gastritis and peptic ulcers: 1. Must avoid substances that stimulate acid secretion, including coffee. 2. Often must take: a. Antacids. b. H2-histamine receptor blockers or proton pump inhibitors. c. If the cause is excessive activity of Helicobacter pylori, antibiotics may also be required. Small Intestine (Fig. 18.9 page 628 & 18.10 page 629) Is that portion of the GI tract between the pyloric sphincter of the stomach and ileocecal valve opening into the large intestine. It has relatively small diameter (hence the name "small") compared to the large intestine. Is the longest part of the GI tract (approximately 3 m or 12 ft) in a living person, but it will measure nearly twice this length in a cadaver when the muscle wall is relaxed. Divided into: 1.The duodenum is the first 20-30 cm extending from the pyloric sphincter. 2.The jejunum is the next 2/5th of the small intestine. 3.The ileum is the last 3/5th of the small intestine which empties into the large intestine through the ileocecal valve. The small intestine Figure 18.9 The small intestine. (a) The regions of the small intestine. (b) A section of the intestinal wall showing the tissue layers, plicae circulares, and villi. The products of digestion are absorbed across the epithelial lining of the intestinal mucosa: 1. Absorption of CH2O, lipids, a.a., Ca++, and Fe occurs primarily in the duodenum and jejunum. 2. Absorption of bile salts, vitamin B12, water, and electrolytes occurs primarily in the ileum. Absorption occurs at a rapid rate as a result of extensive foldings of the intestinal mucosa, which greatly increase its absorptive surface area. The mucosa and submucosa form large folds called plicae circulares. The microscopic folds of mucosa are called villi. Foldings of the apical plasma membrane of epithelial cells are called microvilli. The histology of the duodenum Note the duodenal (Brunner’s) glands. These exocrine glands, unique to the duodenum, extend into the submucosa and produce a bicarbonate-rich alkaline secretion. I’ve learned that most of the things I worry about never happen. Villi and Microvilli (Fig. 18.10 page 629 & Fig. 18.11 page 630) Each villus is a fingerlike fold of mucosa that projects into the intestinal lumen. The villi are covered with columnar epithelial cells, among which are interspersed mucus-secreting goblet cells. The lamina propria, which forms the connective tissue core of each villus, contains numerous lymphocytes, blood capillaries, and a lymphatic vessel called the central lacteal. Absorbed monosaccharides and a.a. enter the blood capillaries. Absorbed fat enters the central lacteal. Epithelial cells at the tips of the villi are continuously exfoliated (shed) and are replaced by cells that are pushed up from the bases of the villi. The epithelium at the base of the villi invaginates downward at various points to form narrow pouches that open through pores to the intestinal lumen. They are called intestinal crypts, or crypts of Lieberkühn. At the bottom of the intestinal crypts in the small (but not the large) intestine are: 1. Paneth cells which secrete antibacterial lysozyme and bactericidal peptides called defensins. 2. Intestinal stem cells which divide by mitosis to replenish themselves and to produce the specialized cells of the intestinal mucosa. Mitosis is estimated to occur 2 times/day. At the top of the crypts, mitosis stops and the cells differentiate into: 1. Secretory cells ( Paneth cells, goblet cells, and endocrine cells). 2. Enterocytes (intestinal epithelial cells). These newly formed cells migrate from the crypts to the tip of the villi, a journey of 3 days. Cells at the tips of the villi then undergo apoptosis and are shed into the lumen. Therefore, the intestinal epithelium is renewed every 4-5 days. Microvilli (also called brush border) are formed by foldings at the apical surface of each epithelial cell membrane. The structure of an intestinal villus Figure 18.10 The structure of an intestinal villus. The figure also depicts an intestinal crypt (crypt of Lieberkühn), in which new epithelial cell are produced by mitosis. Figure 18.11 Electron micrograph of microvilli . Microvilli are evident at the apical surface of the columnar epithelial cells in the small intestine. Microvilli increase the surface area for absorption and also have the brush border digestive enzymes embedded in their plasma membrane. Intestinal Enzymes (Fig. 18.12 & Table 18.1 page 630) The plasma membrane of the microvilli has 2 functions: 1.Provide a large surface area for absorption. 2.Contain digestive enzymes that hydrolyze disaccharides, polypeptides, and other substrates. These brush border enzymes are not secreted into the lumen, but instead remain attached to the plasma membrane with their active sites exposed to the chyme. Fig. 18.12 Location of brush border enzymes. The brush border enzymes (En) are embedded in the plasma membrane of the microvilli in the small intestine. The active sites of these enzymes face the chyme in the lumen, helping to complete the digestion of food molecules. Intestinal Contractions and Motility (Fig. 18.13 page 631) There are 2 major types of contractions occur in the small intestine: 1.Peristalsis: is much weaker in the small intestine than in the esophagus and stomach. Intestinal motility is the movement of chyme through the intestine, is relatively slow, and is due primarily to the fact that the pressure at the pyloric end of the small intestine is greater than at the distal end. 2.Segmentation: is the major contractile activity of the small intestine. It refers to muscular constrictions of the lumen, which occurs simultaneously at different intestinal segments. It serves to mix the chyme more thoroughly. Occurs more frequently in the proximal than in the distal end of the intestine, producing the pressure difference mentioned earlier and helping to move chyme in a forward direction through the small intestine. Figure 18.13 Segmentation of the small intestine. Simultaneous contraction of numerous segment of the intestine help to mix the chyme with digestive enzymes and mucus. االبتسامة انحناءة تستقيم بها كل األمور The Large Intestine (Fig.18.16 page 632 & 18.18 page 633) Extends from the ileocecal valve to the anus, framing the small intestine on three sides. It is divided into: 1. Cecum which is a blind pouch (open at one end) at the beginning of the large intestine. It receives the chyme from the ileum. 2. Ascending colon 3. Transverse colon 4. Descending colon 5. Sigmoid colon 6. Rectum 7. Anal canal 8. Anus is the external opening of the anal canal through which waste material (feces) is excreted. Figure 18.16 The large intestine Figure 18. A photomicrograph of the human appendix. This cross section reveals numerous lymphatic nodules, which function in immunity. Figure 18.18 A radiograph of the large intestine The large intestine is seen after a barium enema has been administered; the haustra are clearly visible. The mucosa of the large intestine contains many scattered lymphocytes and lymphatic nodules and is covered by columnar epithelial cells and mucussecreting goblet cells. The columnar epithelium form crypts but there are no villi in the large intestine and the intestinal mucosa appears flat. The outer surface of the colon bulges outward to form pouches or haustra. Occasionally, the muscularis externa of the haustra may become so weakened that the wall forms a more elongated out-pouching, or diverticulum. Inflammation of one or more of these structures is called diverticulitis. It has little or no digestive function, but it does absorb water and electrolytes from the remaining chyme, as well as several B complex vitamins and vitamin K. Intestinal Microbiota Microorganisms, primarily bacteria, are present in relatively small numbers in the stomach and proximal portion of the small intestine. Their numbers increase in the distal ileum and are greatest in the colon, where and estimated 1014 reside. They represent several thousand different species. They are known collectively as the intestinal microbiota or microflora. In the colon, the intestinal microbiota are comprised mostly of anaerobic bacterial species. The microbiota are usually described as composed of commensal bacteria. Commensalism refers to a relationship where one species benefits and the other is neither benefited nor harmed. Mutualism may better describe the relationship between the intestinal microbiota and their human hosts. Mutualism occurs when both species benefit. Humans provide the bacteria with nutrients and an anaerobic home in the large intestine; and they provide humans with a variety of benefits. Microbes from the mother invades an infant’s intestine during birth, and invasion from the immediate environment during early infancy. This initial colonization affects the composition of the microbiota later in life. This explains why the composition of the intestinal microbiota varies substantially between individuals and shows more similarity among family members than among unrelated people. This microbiota originates at birth and performs a number of physiologically important functions: 1.Production of B vitamins and vitamin K. 2.Fermentation of some indigestible molecules in the chyme that enters the large intestine. 3.Production of short-chain fatty acids (< 5C long as acetate, propionate, and butyrate) which are used for energy by the epithelial cells of the colon, and which aid the absorption of Na+, HCO3-, Ca2+, Mg2+, Fe3+ in the large intestine. 4.The correct balance of the bacterial species in the microbiota reduces the ability of pathogenic species to cause damage and produce such symptoms as diarrhea. 5.There is evidence that a normal intestinal microbiota exerts an anti-inflammatory effect in the gut, and evokes responses that help protect the intestinal epithelium from injury. The intestine is protected from bacteria in its lumen by: 1. Mucus from goblet cells, which prevent most bacteria from reaching the epithelial cells. 2. Secretion of antimicrobial peptides (defensins) by Paneth cells in the crypts. 3. Secretion of IgA antibodies by plasma cells. A normal intestinal microbiota helps to maintain a healthy epithelial barrier that protects deeper tissues, limits inflammatory damage, and promotes the repair of a damaged epithelium. Fluid and Electrolyte Absorption in the Intestine The GI receives about 1.5 L/day from drinking and eating, plus the GI tract secrets 8-10 L/day of fluid into the lumen. This include contributions from the salivary glands, stomach, intestine, pancreas, liver, and gallbladder. The small intestine both secretes and absorbs water accompanying different transport processed, but these are not in balance. The small intestine secretes about 1L/day but absorbs most of the fluid in the chyme. Only about 2 L/day of fluid pass into the large intestine, which absorbs about 90% of this remaining volume, leaving < 200ml of fluid to be excreted in the feces. Absorption of water in the intestine occurs passively as a result of the osmotic gradient created by the active transport of ions. The handling of salt and water transport in the large intestine is made more complex by the fact that the large intestine can secrete, as well as absorb, water. The secretion of water by the mucosa of the large intestine occurs by osmosis as a result of the active transport of Na+ or Cl- out of the epithelial cells into the intestinal lumen. Secretion in this way is normally minor compared to the far greater amount of salt and water absorption, but this balance may be altered in some disease states. Defecation After the electrolytes and water have been absorbed, the waste material that is left passes to the rectum leading to an increase in rectal pressure, relaxation of the internal anal sphincter, and the urge to defecate. If the urge to defecate is denied, feces are prevented from entering the anal canal by the external anal sphincter and thus remaining in the rectum or may even back up into the sigmoid colon. The defecation reflex normally occurs when the rectal pressure rises to a particular level that is determined, to a larger degree, by habit. At this point, the external anal sphincter relaxes to admit feces into the anal canal. During the act of defecation, the longitudinal rectal muscles contract to increase rectal pressure, and the internal and external anal sphincter muscles relax. Excretion is aided by contractions of abdominal and pelvic skeletal muscles, which raise the intraabdominal pressure. The raised pressure helps to push the feces from the rectum, through the anal canal, and out of the anus. I’ve learned that you can’t hide a piece of broccoli in a glass of milk. Liver, Gallbladder, and Pancreas The liver is positioned immediately beneath the diaphragm in the abdominal cavity. The liver is largest internal organ, weighing about 1.3 kg (3.3-4.0 lb) in an adult. The gallbladder, which is 7-10 cm (3-4 in) long, has a pearshape and attached to the inferior surface of the liver. The pancreas, which is about 12-15 cm (5-6 in) long, is located behind the stomach along the posterior abdominal wall Structure of the Liver (Fig. 18.19 page 635) The liver is 1-2 cells thick. The liver cells, called hepatocytes, form hepatic plates that are 1-2 cells thick. The plates are separated from each other by large capillary spaces called sinusoids. The sinusoids have extremely large pores called fenestrae. The sinusoids lack a basement membrane which makes them much more permeable than other capillaries, even permitting the passage of plasma proteins with protein-bound nonpolar molecules, such as fat and cholesterol. The sinusoids also contain phagocytic Kupffer cells. The fenestrae (lack of a basement membrane) and plate structure of the liver provide intimate contact between the hepatocytes and the content of the blood. The liver has an amazing ability to regenerate itself. This regenerative ability is not due to stem cells, but rather to the mitotic division of the remaining hepatocytes. When the original mass is restored, cell division ceases. The same regenerative ability is seen when most toxins or infections cause the hepatocytes to die. For reasons not presently understood, hepatic damage due to alcohol abuse and viral hepatitis can cause liver fibrosis, where there is accumulation of collagen fibers and extracellular matrix. This can lead to cirrhosis, a more serious condition. Figure 18.19 Microscopic structure of the liver. Blood enters a liver lobule through the vessels in a portal triad, passes through hepatic sinusoids, and leaves the lobule through a central vein. The central veins converge to form hepatic veins that transport venous blood from the liver. Hepatic Portal System The products of digestion that are absorbed into blood capillaries in the intestine do not directly enter the general circulation. Instead, this blood is delivered first to the liver. Capillaries in the digestive tract drain into the hepatic portal vein, which carries this blood to capillaries in the liver. It is not until the blood has passed through this second capillary bed that it enters the general circulation through the hepatic vein that drains the liver. The term portal system is used to describe this unique pattern of circulation: Capillaries → Vein → Capillaries → Vein. The liver also receives arterial blood via the hepatic artery. Figure 18.20 The flow of blood and bile in a liver lobule. Blood flows within sinusoids from a portal vein to the central vein (from the periphery to the center of a lobule). Bile flows within hepatic plates from the center to bile ductules at the periphery of a lobule. Liver Lobules (Fig. 18.19 page 635 & 18.20 page 636) The hepatic plates are arranged into functional units called liver lobules. In the middle of each lobule is a central vein. At the periphery of each lobule are branches of the hepatic portal vein and of the hepatic artery, which open into the sinusoids between hepatic plates. Arterial blood and portal venous blood, containing molecules absorbed in the GI tract, thus mix as the blood flows within the sinusoids from the periphery of the lobule to the central vein. The central veins of different liver lobules converge to form the hepatic vein, which carries blood from the liver to the inferior vena cava. Bile is produced by the hepatocytes and secreted into thin channels called bile canaliculi, located within each hepatic plate. These bile canaliculi are drained at the periphery of each lobule by bile ducts, which in turn drain into hepatic ducts that carry bile away from the liver. Blood and bile do not mix in the liver lobules, because blood travels in the sinusoids and bile travels in the opposite direction within the hepatic plates. Enterohepatic Circulation (Fig. 18.21 & Table 18.2 page 637) In addition to the normal constituents of bile, a wide variety of exogenous compounds (drugs) are secreted by the liver into the bile ducts. The liver can "clear" the blood of particular compounds by removing them from the blood and excreting them into the intestine with the bile. Molecules that are cleared from the blood by secretion into the bile are eliminated in the feces (this is analogous to renal clearance of blood through excretion in the urine). Many compounds that are released with the bile into the intestine are not eliminated with the feces; some can be absorbed through the small intestine and enter the hepatic portal blood. These molecules are thus carried back to the liver, where they can be again secreted by hepatocytes into the bile ducts. Compounds that recirculate between the liver and intestine in this way are said to have an enterohepatic circulation (for example: a few grams of bile salts released into the intestine recirculate six to ten times a day, with only about 0.5g of bile salts/day excreted in the feces). Figure 18.21 The enterohepatic circulation. Substances secreted in the bile may be absorbed by the intestinal epithelium and recycled to the liver via the hepatic portal vein. We see the world suffering But still we get Married. That’s LOVE Function of the liver (Table 18.3 page 637) • As a result of its large and diverse enzymatic content and its unique structure, and because it receives venous blood from the intestine, the liver has a wider variety of functions than any other organ in the body. These functions are: 1.Bile Production and Secretion: The liver produces and secretes 250-1500 ml of bile/day. The major constituents of bile are bile pigment (bilirubin), bile salts, phospholipids (mainly lecithin), cholesterol, and inorganic ions. Bilirubin is produced in the spleen, liver, and bone marrow as a derivative of the heme groups (minus the iron) from hemoglobin. (Fig.18.22 page 638). The free bilirubin is not very water-soluble, and thus most is carried in the blood attached to albumin proteins, which can neither be filtered by the kidneys into the urine nor directly excreted by the liver into the bile. The liver can take some the free bilirubin out of the blood and conjugate it with glucuronic acid. Figure 18.22 Simplified pathway for the metabolism of heme and bilirubin. Heme can be formed from the hemoglobin in red blood cells. The iron from the heme group is recycled back to the bone marrow when the heme is converted into biliverdin. Notice that carbon monoxide is produce in this process and, since it is toxic, must be eliminated from the body. The conjugated bilirubin is water-soluble and can be secreted into the bile. The conjugated bilirubin in the bile can enter the intestine where it is converted by bacteria into another pigment called urobilinogen. Derivatives of urobilinogen impart a brown color to the feces. About 30-50% of urobilinogen is absorbed by the intestine and enters the hepatic portal vein. Of the urobilinogen that enters the liver sinusoids, some is secreted into the bile and is thus returned to the intestine in an enterohepatic circulation; the rest enters the general circulation (Fig. 18.24 page 616). The urobilinogen in plasma, unlike free bilirubin, is not attached to albumin. Urobilinogen is therefore easily filtered by the kidneys into the urine, where its derivatives produce an amber color. Bile salts are derivatives of cholesterol that have 2-4 polar groups on each molecule. Figure 18.23 The enterohepatic circulation of urobilinogen. Bacteria in the intestine convert bilirubin (bile pigment) into urobilinogen. Some of this pigment leaves the body in the feces; some is absorbed by the intestine and is recycled through the liver. A portion of the urobilinogen that is absorbed enters the general circulation and is filtered by the kidneys into the urine. The principal bile acids in humans are cholic acid and chenodeoxycholic acid, conjugated with the amino acids glycine or taurine to form the bile salts. In aqueous solutions these molecules "huddle" together to form aggregates known as micelles. Micelles have nonpolar parts located in its central region, and polar groups face water around its periphery. Lecithin, cholesterol, and other lipids in the small intestine enter these micelles, and the dual nature of the bile salts allows them to emulsify fat in the chyme. The liver's production of bile acids from cholesterol is the major pathway of cholesterol breakdown in the body. This amounts to about 1/2 gram of cholesterol converted into bile acids /day. No more than this is required, because about 95% of the bile acids released into the duodenum are absorbed in the ileum by means of specific carriers, and so have an enterohepatic circulation. Bile salts recirculate 6-10 times/day, with only about 0.5 g excreted in the feces. The two major bile acids in humans. Derivatives of cholesterol form the bile salts. Cholic acid Chenodeoxycholic acid Fig.18.24 Bile acids form micelles. (a) Cholic acid, a bile acid. (b) A simplified representation of a bile acid, emphasizing that part of the molecule is polar, but most is nonpolar. (c) Bile acids in water aggregate to form associations called micelles. Cholesterol and lecithin, being nonpolar, can enter the micelles. The bile acids in the micelles serve to emulsify triglycerides (fats and oils) in the chyme. Figure 2.22 The structure of lecithin Lecithin is also called phosphatidylcholine, where choline is the nitrogen- containing portion of the molecule. The circle represents the polar portion and the saw-toothed lines the nonpolar portion of the molecule. Figure 2.23 The formation of a micelle structure by phospholipids such as lecithin The hydrophilic outer layer of the micelle faces the aqueous environment. Detoxification of the Blood The liver’s endothelial cells (lining the sinusoids), Kupffer cells, and dendritic cells have pathogen recognition receptors that recognize PAMPs (pathogenassociated molecular patterns) enabling them to scavenge blood-borne bacteria. The liver can also remove hormones, drugs, and other biologically active molecules from the blood by: a. Excretion of these compounds in the bile. b. Phagocytosis by the Kupffer cells that line the sinusoids. c. Chemical alteration of these molecules within the hepatocytes. Ammonia is a very toxic molecule, produced by deamination of a.a. in the liver and by the action of bacteria in the intestine. Since the ammonia concentration of portal vein blood is four to fifty times greater than that of blood in the hepatic vein, it is clear that the ammonia is removed by the liver. The liver has enzymes needed to convert ammonia into less toxic urea molecules, which are secreted by the liver into the blood and excreted by the kidneys in the urine. Similarly, the liver converts toxic porphyrins into bilirubin, and toxic purines into uric acid. Steroid hormones and many drugs are inactivated in their passage through the liver by modifications of their chemical structures. The liver has enzymes that convert these nonpolar molecules into more polar forms by hydroxylation and by conjugation with highly polar groups such as sulfate and glucuronic acid. Polar derivatives of steroid hormones and drugs are less biologically active and, because of their increased water solubility, are more easily excreted by the kidneys into the urine. 3.Secretion of Glucose, Triglycerides, and Ketone Bodies: The liver helps to regulate the blood glucose concentration by either removing glucose from the blood or adding glucose to it, according to the needs of the body. After a CH2O-rich meal, the liver can remove some glucose from the hepatic portal blood and convert it into glycogen and triglycerides through the processes of glycogenesis and lipogenesis, respectively. During fasting, the liver secretes glucose into the blood. This glucose can be derived from the breakdown of stored glycogen in a process called glycogenolysis, or it can be produced by the conversion of noncarbohydrate (such as amino acids) molecules into glucose in a process called gluconeogenesis ( Fig.5.5 page 112). The liver also contains enzymes required to convert free fatty acids into ketone bodies (ketogenesis), which are secreted into the blood in large amounts during fasting. These processes are controlled by hormones. Figure 5.10 Glycogenesis and glycogenolysis. Blood glucose entering tissue cells is phosphorylated to glucose 6-phosphate. This intermediate can be metabolized for energy in glycolysis, or it can be converted to glycogen (1) in a process called glycogensis. Glycogen represents a storage from of carbohydrates that can be used as a source for new glucose 6-phosphate (2) in a process called glycgenolysis. The liver contains an enzyme that can remove the phosphate from glucose 6-phosphate; liver glycogen this serves as a source for new blood glucose. 4. Production of Plasma Proteins: Plasma albumin and most of the plasma globulins (with the exception of immunoglobulins, or antibodies) are produced by the liver. Albumin constitutes about 70% of the total plasma protein and contributes most to the colloid osmotic pressure of the blood. The globulins produced by the liver have a wide variety of functions: a. Transport of cholesterol and triglycerides. b. Transport of steroid and thyroid hormones. c. Inhibition of trypsin activity. d. Blood clotting. Clotting factors I (fibrinogen), II (prothrombin), III, V, VII, IX, and XI, as well as angiotensinogen, are all produced by the liver. On an old man’s shirt was Written a cute sentence “I Am Not 60 Years Old--I am Sweet 16 with 44 Years Experience.” That’s ATTITUDE Gallbladder (Fig. 18.25 page 641) Is a saclike organ attached to the inferior surface of the liver. It stores and concentrates bile, which drains to it from the liver by way of the bile ducts, hepatic ducts, and cystic duct respectively. Bile is a yellowish green fluid containing bile salts, bilirubin, cholesterol, and other compounds. Contraction of the muscularis layer of the gallbladder ejects bile through the cystic duct into the common bile duct, which conveys bile into the duodenum. Bile is continuously produced by the liver and drains through the hepatic and common bile ducts to the duodenum. Figure 18.25 Pancreatic juice and bile are secreted into the duodenum. The pancreatic duct joins the common bile duct to empty its secretions through the duodenal papilla into the duodenum. The release of bile and pancreatic juice into the duodenum is controlled by the sphincter ampulla (sphincter of Oddi). Figure 18.26 Gallstones. (a) A radiograph of a gallbladder that contains gallstones (biliary calculi). (b) A posterior view of a gallbladder that has been surgically removed (cholecystectomy) and cut open to reveal its gallstones. (Notice their size relative to that of a dime.) Pancreas (Fig. 18.25 page 641 & Fig. 18.27 page 642) Is a soft, glandular organ that has both exocrine and endocrine functions. The endocrine function is performed by clusters of cells called the pancreatic islets, or islets of Langerhans that secrete the hormones insulin and glucagons into the blood. As an exocrine gland, the pancreas secretes pancreatic juice through the pancreatic duct into the duodenum. Within the lobules of the pancreas are the exocrine secretory units, called acini. Each acinus consists of a single layer of epithelial cells surrounding a lumen, into which the constituents of pancreatic juice are secreted. Figure 18.27 Pancreatic juice and bile are secreted into the duodenum. (a) A photomicrograph of the endocrine and exocrine portion of the pancreas. (b) An illustration depicting the exocrine pancreatic acini, where the acinar cells produce inactive enzymes stored in zymogen granules. The inactive enzymes are secreted by way of a duct system into the duodenum. Pancreatic juice It contains: a. Bicarbonate, which is secreted by cells that line the ductules, and it is produced from CO2 that diffuses into the cells from the blood. This occurs through the action of carbonic anhydrase in forming carbonic acid, and results in the secretion of H+ into the blood as HCO3- is secreted into the pancreatic juice. Secretion of HCO3- from the ductile cells into the lumen is accompanied by movement of Cl- in the opposite direction (Fig. 18.29 page 620). b. Amylase, which digests starch. c. Trypsin, which digests protein. d. Lipase, which digests triglycerides. e. Other enzymes listed in table 18.4 page 643. Fig. 18.28 Secretion of bicarbonate into pancreatic juice. Cells of the pancreatic duct take in CO2 from the blood and use it to generate carbonic acid. This dissociates into HCO3- and H+. The HCO3- is secreted into the lumen of the duct by a carrier that exchanges it for Cl- . The Cl- then leaks passively back into the lumen through a different chloride channel. The complete digestion of food molecules in the small intestine requires the action of both pancreatic enzymes and brush border enzymes. Most pancreatic enzymes are produced as inactive molecules, or zymogens, so that the risk of self-digestion within the pancreas is minimized. The inactive form of trypsin, called trypsinogen, is activated within the small intestine by the catalytic action of the brush border enzyme enterokinase (also called enteropeptidase). Enterokinase converts trypsinogen to active trypsin. Trypsin activates the other zymogens of pancreatic juice by cleaving off polypeptide sequences that inhibit the activity of these enzymes. (Fig. 18.29 page 643). Figure 18.29 The activation of pancreatic juice enzymes. The pancreatic protein-digesting enzyme trypsin is secreted in an inactive from known as trypsinogen. This inactive enzyme (zymogen) is activated by a brush border enzyme, enteokinase (EN), located in the cell membrane of microvilli. Active trypsin in turn activates other zymogens in pancreatic juice. I’ve learned that you can get by on charm for about fifteen minutes. After that, you’d better know something. Regulation of the Digestive System Neural and endocrine control mechanisms modify the activity of the digestive system. The sight, smell, or taste of food can stimulate salivary and gastric secretions via activation of the vagus nerve, which helps to "prime" the digestive system in preparation for a meal. Stimulation of the vagus, in this case, originates in the brain and is a conditioned reflex. The vagus nerve is also involved in the reflex control of one part of the digestive system by another (these are "short reflexes," which do not involve the brain. The GI is both an endocrine gland and a target for the action of various hormones (Table 18.5 page 645). I. Regulation of Gastric Function Gastric motility and secretion are, to some extent, automatic. For example, waves of contraction that serve to push chyme through the pyloric sphincter are initiated spontaneously by pace-setter cells in the great curvature of the stomach. The secretion of HCl from parietal cells and pepsinogen from chief cells can be stimulated in the absence of neural and hormonal influences by the presence of cooked or partially digested protein in the stomach. This action involves other cells in the gastric mucosa, including the G cells (that secrete the hormone gastrin); the ECL cells, which secrete histamine; and the D cells which secrete somatostatin. The effect of autonomic nerves and hormones are superimposed on this automatic activity. This extrinsic control of gastric function is divided into 3 phases : ( Table 18.6 page 645) •The cephalic phase. •The gastric phase. •The intestinal phase. Figure 18.31 The regulation of gastric acid secretion. The presence of amino acids in the stomach lumen from partially digested proteins stimulates gastrin secretion. Gastrin secretion from G cells is also stimulated by vagus nerve activity. The secreted gastrin then acts as a hormone to stimulate histamine release from the ECL cells. The histamine, inturn, acts a paracrine regulator to stimulate the parietal cells to secrete HCl. II. Regulation of intestinal function Enteric Nervous System The neurons and glial cells of the enteric nervous system are organized into ganglia that are interconnected by 2 plexuses: 1. The outer myenteric (Auerbach's) plexus is found along the entire length of the GI tract. 2. The inner submucosal (Meissner's) plexus is located only in the small and large intestine. Peristalsis is regulated by the enteric nervous system. A bolus of chyme stimulates intrinsic afferent that activate enteric interneurons, which in turn stimulate motor neurons. These motor neurons innervate both smooth muscle cells and interstitial cells of Cajal, where they release excitatory and inhibitory neutotransmitters. Smooth muscle contraction is stimulated by the neurotransmitters ACh and substance P above the bolus, and smooth muscle relaxation is stimulated by nitric oxide, vasoactive intestinal peptide (VIP), and ATP below the bolus (Fig. 18.31 page 648). Figure 18.31 The enteric nervous system coordinates peristalsis. Peristalsis is produced by local reflexes involving the enteric nervous system. Notice that the enteric nervous system consists of motor neurons, interneurons, and sensory neurons. (NO=Nitric oxide; VIP= Vasoactive intestinal peptide.) Paracrine Regulators of the Intestine ECL cells (enterochromaffin-like cells) of the intestinal mucosa secrete serotonin (5-hydroxytryptamine) in response to the stimuli of pressure and various chemicals. Serotonin then stimulates neurons that terminate in the muscularis can stimulate contractions. Those neurons that terminate in the intestinal crypts can stimulate the secretion of salt and water into the lumen. The ECL cells have also been shown to produce another paracrine regulator, termed motilin, which stimulates contraction in the duodenum and stomach antrum. Guanylin is a paracrine regulator produced by the ileum and colon. Guanylin stimulate the intestinal epithelial cells to secrete Cl- and water and to inhibit their absorption of Na+. These actions increase the amount of salt and water lost from the body in the feces. Uroguanylin has been found in urine, produced by the intestine and may function as a hormone that stimulates the kidneys to excrete salt in the urine. Intestinal Reflexes There are several intestinal reflexes that are controlled locally, by means of the enteric nervous system and paracrine regulators, and extrinsically through the actions of the nerves and hormones. These reflexes include: 1.The gastroileal reflex, in which increased gastric activity causes increased motility of the ileum and increased movements of chyme through the ileocecal sphincter. 2.The ileogastric reflex, in which distension of the ileum causes a decrease in gastric motility. 3.The intestino-intestinal reflexes, in which overdistension of one intestinal segment causes relaxation throughout the rest of the intestine. الحياة تعلمك الحب والتجارب تعلمك من تحب III. Regulation of Pancreatic Juice and Bile Secretion The arrival of chyme into the duodenum stimulates the intestinal phase of gastric regulation and, at the same time, stimulates reflex secretion of pancreatic juice and bile. The entry of new chyme is thereby retarded by the inhibitory effects of neural reflexes and enterogastrone, allowing time for the previous load of chyme in the duodenum to be digested with the aid of pancreatic juice enzymes and bile. The secretion of pancreatic juice and bile is stimulated both by neural reflexes initiated in the duodenum and by secretion of the duodenal hormones CCK and secretin. Trophic Effects of Gastrointestinal Hormones Gastrin secreted by the pyloric mucosa may exert stimulatory, or trophic, effects on the gastric mucosa. The structure of the gastric mucosa is dependent upon the effects of gastrin. The structure of the acinar (exocrine) cells of the pancreas is dependent upon the trophic effects of CCK. Since neural reflexes appear to be capable of regulating digestion, perhaps the primary function of the GI hormones is trophic, that is, maintenance of the structure of their target organs. Digestion and Absorption of Carbohydrates, Lipids, and Proteins (Table 18.7 page 650) The caloric (energy) value of food is derived mainly from its content of CH2O, lipid, and proteins. In the average diet, carbohydrates account for approximately 50% of the total calories, and protein accounts for 11%-14%, and the lipids make up the balance. These food molecules consist primarily of long combinations of subunits (monomers) that must be digested by hydrolysis reactions into free monomers before absorption can occur. I. Digestion and Absorption of Carbohydrates. Most CH2O are ingested as starch. Starch is a long polysaccharide of glucose in the form of straight chains with occasional branching. The most commonly ingested sugars are sucrose(table sugar, a disaccharide of glucose and fructose) and lactose (milk sugar, a disaccharide of glucose and galactose). The digestion of starch begins in the mouth with the action of salivary amylase. Amylase cleaves some of the bonds between adjacent glucose molecules, but most people don't chew their food long enough for sufficient digestion to occur in the mouth. The digestive action of salivary amylase stops some time after the swallowed bolus enters the stomach because this enzyme is inactivated at the low pH of gastric juice. Digestion of starch occurs mainly in the duodenum as a result of the action of pancreatic amylase (Fig. 18.32 page 651). Figure 18.32 The action of pancreatic amylase. Pancreatic amylase digests starch into maltose, maltriose, and short oligosaccharides containing branch points in the chain of glucose molecules. Pancreatic amylase cleaves the straight chains of starch to produce the disaccharide maltose and the trisaccharide maltriose. Pancreatic amylase cannot hydrolyze the bond between glucose molecules at the branch points in the starch. As a result, short, branched chains of glucose molecules called oligosaccharides are released together with maltose and maltriose by the activity of this enzyme. Maltose, maltriose, and oligosaccharides are hydrolyzed to their monosaccharides by brush border enzymes located on the microvilli of the epithelial cells in the small intestine. The brush border enzymes also hydrolyze the disaccharides sucrose and lactose into their component monosaccharides. Monosaccharides are then moved across the brush border membrane by secondary active transport. One glucose molecule is cotransported with 2Na+ into the epithelial cell cytoplasm. The glucose then moves by facilitative diffusion through GLUT transporters across the basolateral membrane into the interstitial fluid and capillary blood in the villus. The absorbed monosaccharides enter the hepatic portal vein and travel to the liver. The absorption of Na+ that accompanies the absorption of glucose ( and other nutrients) results in the simultaneous movement of Cl- due to potential difference created by Na+ transport. Water follows the NaCl through the paracellular route between epithelial cells and is absorbed into the blood along with the glucose and NaCl. II. Digestion and absorption of Proteins. Protein digestion begins in the stomach with the action of pepsin. Some a.a. are liberated in the stomach, but the major products of pepsin digestion are short-chain polypeptides. Pepsin digestion helps to produce a more homogenous chyme, but it is not essential for the complete digestion of protein (even in people with total gastrectomies) that occurs in the small intestine. Most protein digestion occurs in the duodenum and jejunum. The pancreatic enzymes trypsin, chymotrypsin, and elastase cleave peptide bonds in the interior of the polypeptide chains, and are grouped together as endopeptidases. Enzymes that remove a.a from the ends of polypeptide chain are called exopeptidases. Exopeptidases include the pancreatic juice enzyme carboxypeptidase, which removes a.a. from the carboxyl-terminal end of polypeptide chains, and the brush border enzyme aminopeptidase, which cleaves a.a. from the aminoterminal end of polypeptide chains. As a result of the action of these enzymes, polypeptides chains are digested into free a.a., dipeptides, and tripeptides. The free a.a. are absorbed across the brush border membrane by different secondary active transport carriers, most of which Cotransport the a.a with Na+. The dipeptides and tripeptides enter epithelial cells by the action of a single membrane carrier. This carrier functions in secondary active transport using a H+ gradient to transport dipeptides and tripeptides into the cell cytoplasm. Within the cytoplasm, the dipeptides and tripeptides are hydrolyzed into free a.a., which move across the basolateral membrane by facilitated diffusion to the interstitial fluid and then to capillary blood. (Fig.18.33 page 651). The absorbed a.a. enter the blood delivered to the liver by the hepatic portal vein. Newborn babies appear to be capable of absorbing a substantial amount of undigested proteins (hence they can absorb some Abs from their mother's first milk). In adults only free a.a. enter the portal vein. Foreign food protein, which would be very antigenic, does not normally enter the blood. Except the protein toxin that causes botulism, produced by the bacterium Clostridium botulinum. This protein is resistant to digestion and is thus intact when it is absorbed into the blood. Figure 18.33 The digestion and absorption of proteins. Polypeptide chains of proteins are digested into free amino acids, peptides, and tripeptides by the action of pancreatic juice enzymes and brush border enzymes. The amino acids, dipeptides, and tripeptides enter duodenal epithelial cells. Dipeptides and epithelial cells, and these tripeptides are hydrolyzed into free amino acids within the products are secreted into capillaries that carry them to the hepatic portal vein. ليس في العالم وسادة أنعم من حضن أمي III. Digestion and absorption of Lipids The salivary glands and stomach of neonates produce lipases. In adults very little lipid digestion occurs until the lipid globules in chyme arrive in the duodenum. The arrival of lipids in the duodenum serves as a stimulus for the secretion of bile. In a process called emulsification, bile salts micelles are secreted into the duodenum and act to break up the fat droplets into tiny emulsification droplets of triglycerides. Emulsification is not chemical digestion, the bonds joining glycerol and fatty acids are not hydrolyzed by this process. Digestion of Lipids The emulsification of fat aids digestion because the smaller and more numerous emulsification droplets present a greater surface area than the unemulsified fat droplets that originally entered the duodenum. Fat digestion occurs at the surface of the droplets through the enzymatic action of pancreatic lipase. Lipase is aided in its action by a protein called colipase. Colipase is secreted by the pancreas. Colipase coats the emulsification droplets and anchors the lipase enzyme to them. Lipase hydrolyzes fats and removes 2 of the 3 fatty acids from each triglyceride molecule and thus liberates free fatty acids and monoglycerides (Fig.18.34 page 652). Figure 18.34 The digestion of triglycerides. Pancreatic lipase digests fat (triglycerides) by cleaving off the first and third fatty acids. This produces free fatty acids and monoglycerides. Saw tooth lines indicate hydrocarbon chains in the fatty acids. Phospholipase A digests phospholipids such as lecithin into fatty acids and lysolecithin (the remainder of the lecithin molecule after the removal of 2 fatty acids). Free fatty acids, monoglycerides, and lysolecithin derived from the digested lipids are more polar than the undigested lipids. They quickly enter the micelles of bile salts, lecithin, and cholesterol from the bile to form "mixed micelles“ in the duodenum (Fig. 18.35 page 652). The mixed micelles then move to the brush border of the intestinal epithelium where absorption occurs. Figure 2.22 The structure of lecithin Lecithin is also called phosphatidylcholine, where choline is the nitrogen- containing portion of the molecule. The circle represents the polar portion and the saw-toothed lines the nonpolar portion of the molecule. Figure 2.23 The formation of a micelle structure by phospholipids such as lecithin The hydrophilic outer layer of the micelle faces the aqueous environment. Fig. 18.35 Fat digestion and emulsification. The three steps indicate the fate of fat in the small intestine. The digestion of fat (triglycerides) releases fatty acids and monoglycerides, which become associated with micelles of bole salts secreted by the liver. Absorption of Lipids Free fatty acids, monoglycerides, and lysolecithin can leave the micelles and pass through the membrane of the microvilli to enter the intestinal epithelial cells. These products are used to resynthesize triglycerides and phospholipids within the epithelial cells. This process is different from the absorption of a.a. and monosaccharides, which pass through the epithelial cells without being altered. Triglycerides, phospholipids, and cholesterol are then combined with protein inside the epithelial cells to form small particles called chylomicrons. Chylomicrons are tiny lipid and protein combinations. Chylomicrons are secreted into the central lacteals (lymphatic capillaries) of the intestinal villi (Fig.18.36 page 653). Absorbed lipids thus pass through the lymphatic system, eventually entering the venous blood by way of the thoracic duct. Chylomicrons in the blood after a fatty meal turn, otherwise clear, blood plasma cloudy. Figure 18.36 The absorption of fat. Fatty acids and monoglycerides from the micelles within the small intestine are absorbed by epithelial cells and converted intracellularly into triglycerides. These are then combined with protein to form chylomicrons, which enter the lymphatic vessels (lacteals) of the villi. These lymphatic vessels transport the chylomicrons to the thoracic duct, which empties them into the venous blood (of the left subclavian vein). Transport of Lipids in the Blood. Once the chylomicrons in the blood, they acquire a protein constituent, or apolipoprotein, called ApoE (Fig.13.32 page 438). This allows the chylomicrons to bind to receptor proteins for ApoE located on the plasma membrane of capillary endothelial cells in the muscles and adipose tissue. The triglycerides content can then be digested by the enzyme lipoprotein lipase, which is also bound to the endothelial cell plasma membrane. The hydrolysis of triglyceride releases free fatty acids that circulate in the plasma attached to albumin and are used by skeletal muscles for energy and by adipose tissue for the synthesis of stored fat. After the triglyceride content of the chylomicrons has bee removed, the remaining remnant particles containing cholesterol is released and travels in the circulation until it is taken up by the liver. Fig.13.32 page 438. Structure of a lipoprotein. There is a core of nonpolar triglycerides and cholesterol esters coated by proteins (apolipoproteins), phospholipids, and some free cholesterol. Cholesterol and triglycerides produced by the liver are combined with other apolipoproteins and secreted into the blood as very-low-density lipoproteins (VLDLs), which deliver triglycerides to different organs. Removing triglycerides from VLDL particles results in low-density lipoproteins (LDLs), which transport cholesterol to various organs, including blood vessels. This can contribute to the development of atherosclerosis. Excess cholesterol is returned from these organs to the liver attached to highdensity lipoproteins (HDLs) (Table 18.8 page 654). The HDL particles bind to receptors in the blood vessel wall and take up phospholipids and free cholesterol. An enzyme bonds the free cholesterol to the phospholipids, producing cholesterol esters. Since the cholesterol esters are very hydrophobic, they move to the center of the HDL particle, enabling the particle to continue taking up free cholesterol from the cells of the blood vessel. After the HDL particle is fully loaded with cholesterol. It detaches from the vessel wall and travels to the liver to unload its cargo of cholesterol. As a result of this activity, a high ratio of HDL-cholesterol to total cholesterol is believed to afford protection against atherosclerosis. اكثر الناس كذبا أكثرهم الحديث عن نفسه I’ve learned that if love isn’t taught at home, it’s difficult to learn it anywhere else.