* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Modern Atomic Theory Notes Sheet
X-ray photoelectron spectroscopy wikipedia , lookup
Particle in a box wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
Double-slit experiment wikipedia , lookup
Atomic orbital wikipedia , lookup
Astronomical spectroscopy wikipedia , lookup
Electron configuration wikipedia , lookup
Tight binding wikipedia , lookup
Hydrogen atom wikipedia , lookup
Matter wave wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
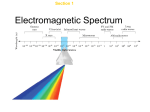
Chemistry Notes/Diagrams Name: _______________________________________ Period: _____ Modern Atomic Theory Notes Flame Test Demo Notes/Diagrams Observations: Why do you think each flame was a different color? Additional Notes/Examples Notetaking Problems with Rutherford’s Atomic Theory: Rutherford’s atom = Problems: Early 1900s: Scientists observed: What did the analysis of the emitted light reveal? Wave Nature of Light: electromagnetic(EM) radiation = includes: all EM waves travel at speed of light: Why do different types of EM waves have different amounts of energy? Wave Nature of Light Continued: frequency is a measure of high frequency waves pass through the fixed point more often than low frequency waves all electromagnetic waves travel at the same speed: the electromagnetic spectrum includes Visible Light: part of the electromagnetic spectrum that we can see What makes up white light? continuous spectrum = Which color of light has the lowest energy? Which color of light has the most energy? Light: Particle or Wave? Wave model of light cannot explain how light interacts with matter Light as a Particle : Atomic Emission Spectra every element emits EM waves of different energy, and so produces different colors when the emitted light is passed through a prism the colors that appear are known as the element’s: Light as a Particle and Emission Spectra: Every element has a unique: The atomic emission spectra phenomenon is a key piece of evidence that light acts like a particle the wave model cannot explain how light interacts with matter ex: why do heated objects only emit certain colors of light? Max Planck: German physicist – 1900 studied: conclusion: quantum = Photoelectric Effect: light of certain minimum frequency causes electrons to be ejected from the surface of metals What happens if the intensity of the light increases? What happens if the frequency of the light increases? used in: Photons: Albert Einstein in 1905 proposed: light is: What are photons? Bohr Model of the Atom: What did scientists think that the element’s atomic emission spectrums would be like? What did Bohr propose for Hydrogen atoms? lowest state = What did Bohr say about Hydrogen’s single electron? higher orbit = What do the lines of hydrogen’s emission spectrum result from? Energy Absorption: When atoms absorb energy, their electrons become “excited” and temporarily move to a higher energy state where they become unstable. They must eventually return to their lowest available energy level known as the ground level. Bohr Model of the Atom Continued Said Hydrogen’s single electron could only be in certain allowed orbits around the nucleus higher orbit = theorized that electrons existed in distinct orbitals or energy levels around the nucleus, and it took an exact amount of energy or quanta to move an electron from one orbital to another, which accounted for the specific light spectra of each element Why was Bohr’s Model abandoned? Electrons as Waves: Bohr’s model only explained Hydrogen, did not explain the chemical behavior of all the other elements Louis de Broglie – 1924 proposed: derived equation that shows all particles have wave characteristics Heisenberg Uncertainty Principle: Werner Heisenberg – German physicist concluded: principle: Schrodinger Wave Equation: Erwin Schrodinger – Austrian physicist 1926 derived equation that treated hydrogen atom’s electron as a wave What did his equation limit electron’s energy to? Quantum Mechanical Model of the Atom: combines de Broglie, Heisenberg, and Schrodinger’s work into one model of the atom can describe: calculates: Atomic Orbitals: definition: assigned numbers: principal quantum number (n) = energy sublevels - Four Principal Energy Levels Principal Quantum Number (n) 1 2 3 4 Sublevels (types of orbitals) present Number of orbitals related to sublevel Total number of orbitals related to principal energy level (n2)