* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Light and Atoms
Survey
Document related concepts
Transcript
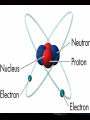
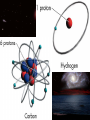
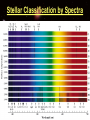
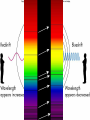
Introduction to Astronomy • Announcements – HW #1 due Wednesday 06/18/2008 – Course Reserves Project Details Project 1: Homemade Spectroscope • Chapter 4 in textbook • In this project, you will build a simple spectroscope from a cardboard tube, aluminum foil, and a grating (which will be supplied) • Construction details can be found at the end of Chapter 4 (pg. 144) • You will sketch the spectra you see (more on this later) from: – Fluorescent light – Mercury/Sodium vapor streetlight – Ordinary incandescent light bulbs – The blue sky (DO NOT LOOK AT THE SUN!) – Flames (use gas-burning stove, add salt to see sodium emission lines and copper wire to see green copper emission lines) – Extra credit for any other sources you want • This writeup must include a picture of your spectroscope. You will keep the real thing. Project 2: Moon Observation • Chapter 6 in textbook • Look at the Moon on an evening when it is nearly full. Make a sketch of the light and dark markings that you see on its surface with the naked eye. • Then observe the Moon with binoculars or through a telescope (PDO is helpful here) and make an enlarged sketch that shows more detail. Mark & identify a few of the craters you can see. • Estimate the diameter of these craters from the knowledge that the Moon’s radius is about 1000 miles (1700 km). How big is the largest crater you can see compared to the size of Logan? Can you you see any lunar rays? If so, sketch them on your drawing. How long are the rays? • Can you mark the landing sites where humans have touched-down? • SHOW ALL STEPS OF YOUR WORK!!! Project 3: Solar Observation • Chapter 11 in textbook • NEVER LOOK DIRECTLY AT THE SUN WITH THE NAKED EYE, OR THROUGH BINOCULARS/TELESCOPE!!!!!!!!! • Measure the diameter of the Sun. – Take a piece of thin, dark cardboard and put a small hole in it. Hold it about 1 meter (3 feet) from a piece of white paper so that a small image of the sun appears on the paper. – Carefully measure the distance (d) between the cardboard and the piece of paper and the size of the Sun’s image (s) on the paper. – On a separate piece of paper, draw two straight lines that cross with a small angle between them (see figure) • Draw two small circles between the lines as shown in the figure. Convince yourself that if D is the distance to the sun (1 AU), and S is the Sun’s diameter, then S/D = s/d – s = size of Sun’s image – d = distance between paper and cardboard • Look up the value of D, then solve for S – Does it agree with the value in table 11.1? • SHOW ALL YOUR WORK!!! Light & Atoms Light & Atoms What is Newton holding? What were the results of this experiment? Properties of Light • Wave-particle duality “Light is a wave on Monday, Wednesday, and Friday, and a particle on Tuesday, Thursday, and Saturday. On Sunday, we have to think about it… “ – Light has wave-like properties, and particlelike properties, depending on the type of observation… – Weird, right? – Analogy: you are wearing a hat. Two people observe you from different positions, but only the one wearing glasses sees the hat… • Wave-like – Interference, diffraction – Like overlapping water waves… • Particle-like – Photoelectric effect – Like a game of marbles… The Schizophrenic Photon • Interference cannot be described by the particle model, and the photoelectric effect cannot be explained by the wave model – But we have observed both! Similarities This “sine”-wave goes on forever in both directions, so it is hard to pinpoint the exact “location” of the wave… Is the energy this wave carries here? A particle, on the other hand, is very localized, so it has a well-defined position… or here? or here? But adding many different waves gives a very localized “wave-packet”… …and these wavepackets behave a lot like particles! Quantum Mechanics calls this the “wavefunction” of the particle, and describes the likelihood that the particle can be found at various positions. Less likely (but still possible) to be here More likely to be here We will usually use the wave model of light from here on out… …but we’ll briefly revisit the photon model when we talk about CCDs in the next chapter Properties of Light • Color – Not physical, all a psychological construct to help the brain sort out different wavelengths of visible light English physicist John Dalton (1766-1844), – λred = 700 nm – λblue = 400 nm – 1 nm = 10-9 m worked on colored shadows, color blindness when he discovered pink flowers appear blue to him… He became obsessed with trying to discover the cause of color-blindness, so he arranged for his doctor to REMOVE ONE OF HIS EYES, so Dalton himself could dissect it to look for blue fluid inside that would cause his condition! Characterizing Light as a Wave • Self-sustaining electric and magnetic vibrations Characterizing Light as a Wave • Wavelength – Distance between successive “crests” or “troughs” of the wave Characterizing Light as a Wave • Frequency – Imagine you are standing next to a traveling light wave (or water wave, if you prefer) that passes you… – How many peaks pass you in 1 second? – Frequency of light = Speed of Light = c Wavelength λ – Speed of Light, c = 3.0 x 108 m/s Properties of Light • “White” light – Mixture of all visible colors – Why doesn’t mixing paint of all colors produce white paint? • Chemical reactions due to pigment The Visible Spectrum Our eyes are sensitive only to an EXTREMELY narrow range of light waves “Visible” or “Optical” light The Electromagnetic Spectrum • Visible light constitutes a tiny, tiny fraction of the whole range of light • Our eyes are only sensitive to visible light, but other types of “light” are all around us… – Radio waves, TV waves, cellphone signals, body heat… – What would the world be like if you could see at radio wavelengths? The EM Spectrum on Earth • Radio – Music, television programs encoded into longwavelength waves – Wireless & bluetooth devices – Communications • Infrared (IR) – Distinguish between hot and cool objects – Heat lamps at fast-food places & cafeterias – Nerves in skin register this type of light as heat • Visible – Everything we can physically see – Light bulbs – Reflected sunlight (on Earth) – Color • Ultraviolet (UV) – Suntanning • Skin cells containing melanin produce Vitamin D when they absorb UV light – Snow blindness – Blacklights & security “watermarks” • X-Ray – Medical, dental X-rays – Dock scanning equipment – By-product of atomic/nuclear detonation • Gamma-Ray (γ) – Highest energy – Dock scanning equipment – Radiation pasteurization • Some normal perishables (meat, milk, fruits & vegetables, etc) can be kept fresh (unrefrigerated) for weeks with a healthy dose of radiation to kill off anything nasty. – Atomic/nuclear weaponry The EM Spectrum in Space • Radio: Pulsars, star remnants • Microwaves: Cold interstellar clouds, cosmic background radiation • IR: Young stars, planets, dust • Visible: Stars, the sun • UV: Hot, bright stars • X-Ray: Collapsed stars, black holes • γ-Ray: Active galaxies, GRBs The EM Spectrum • All these different types of light are the SAME phenomena – Self-sustaining vibrations of electric and magnetic energy • The shape of these vibrating energies determines if the light is IR, UV, visible, etc… • Energy carried by light wave of wavelength, λ: – Energy = hc / λ • Which carries more energy? – Red light or Blue light ? – Blue light or X-rays ? – Infrared light or radio waves ? – Gamma rays or Ultraviolet waves ? Properties of Light • Temperature – Hot objects emit light (electric stove, an iron worked by a blacksmith) – Hotter objects emit shorter-wavelength light • Wien’s Law (pronounced ‘Veen’) – – – – Cool stove, black element A little hotter, red element A little hotter, yellow element Very hot, white element • Wien’s Law – Temperature = constant λmax – One of most important tools for astronomers to measure temperature of stars, planets, galaxies, etc… Wien’s Law • Example – Someone states that because an apple looks red, it must be emitting red light. Fortunately, you have taken USU 1040 and know that person is full of it. How would you show them? – We can assume the wavelength of the red light is ~ 700 nm – Using Wien’s Law, we can calculate the temperature that the apple must have in order to emit mostly red light… • We get Temperature = 7000 °F !!! • Therefore, the apple clearly doesn’t EMIT the red light, so it must only REFLECT it. The Atom The Atom • Protons, Neutrons, and Electrons • “Planetary” model of the atom – Negatively-charged electrons orbit positively-charged nucleus – Electromagnetic force holds atom together – Typical size ~ 10-10 m = 1 ten-billionth of a meter • About X times smaller than the width of a human hair – X ~ 500,000 • Planetary model is easy way to visualize atoms – But it is ultimately wrong! – Accelerated charges radiate photons (light energy) • Therefore, an orbiting electron would constantly lose energy (accelerated by centripetal force) and move to progressively lower orbits – Imagine the International Space Station in orbit… • Ultimately, it would spiral in to the nucleus and the atom would destroy itself. • Why is this clearly incorrect? Quantized Atoms • Electrons only allowed to orbit at certain, discrete distances – Painter on scaffold – Developed from theory that even electrons have wave-like properties (like light) • “matter waves” • ONLY at small scales – a person walking through a door does not diffract (spread out) into multiple people. – Ice cubes do not suddenly teleport out of your glass and into your pocket A fundamental principle of Quantum Mechanics: The electron does not orbit the nucleus. It can be anywhere in the electron “cloud”, but we can’t know precisely where until we measure it Origin of Light & Spectra • Electrons are not confined to single orbits. • They can move to higher or lower orbits with different energies, under the right circumstances. • Spring analogy – Imagine proton and electron are connected by a spring. • To move them further apart, must supply energy to stretch spring • To move them closer together, some energy from stretched spring is released as the spring de-stretches • Analogy – Fast lane & slow lane highway • Merging into fast lane REQUIRES energy • Merging into slow lane GIVES UP energy – Same for electrons jumping from one orbit to another • Defines EMISSION & ABSORPTION of light…. • Emission of light energy = de-stretching the spring • Absorption of light energy = stretching the spring • Conservation of Energy – Rules the Universe, you will NEVER break this law. – Energy of emitted light = difference in energy between upper and lower levels – Difference between energy of upper & lower level = energy of absorbed light ( if NOT equal, NO absorption occurs) HOW is light emitted? • The positively-charged nucleus and the negatively-charged electrons form a system with some amount of “stored” electrical energy – Like a battery, positive and negative terminals • If an electron moves to a lower orbit, closer to the nucleus, it creates an electrical disturbance in the system • A fundamental principle of electromagnetism is that an electric disturbance creates a magnetic disturbance, and vice versa – Maxwell’s Equations • The electrical disturbance produced by the electron moving down to a lower orbit creates a magnetic disturbance, which creates an electric disturbance, which creates a magnetic disturbance, ad infinitum – Viola! A self-sustaining vibration of electric and magnetic energy = Light ! Use in Astronomy • Because we cannot directly measure astronomical sources (with a probe, e.g.), we must analyze the light we get from them – “Spectroscopy” – Because the light we receive comes from the very hot atoms in a star, we expect that some properties of the light can tell us about what atom(s) emitted or absorbed it… • Yes, we can tell a whole lot just from light! Emission Spectra • Produced when electrons move from higher energy orbits to lower energy orbits – Emitting light in the process • Because only certain orbits are allowed, only certain transitions are allowed, therefore only certain wavelengths of light are observed. • Different atoms have different sets of allowed electron orbits, so different atoms produce different emission spectra. – Not too long ago, it was thought that all atoms emitted the same light…triumph of quantum mechanics that it was able to describe the different spectra observed… Absorption Spectra • Now, suppose we shine a light through a cloud of Hydrogen gas – The light that matches the energy difference between the upper and lower levels of a Hydrogen atom will be absorbed by that atom, while other wavelengths will pass unaffected. – This causes the spectrum to contain all normal colors, but with dark bands at the absorbed wavelengths • “Absorption spectrum” • Absorption lines appear at the same wavelengths as emission lines, for a given element. • Emission spectra tell us about how hot an object is, and what it is made of. • Absorption spectra tell us about what lies between us and an object. Announcements • Homework #1 due tomorrow • First Project Due 30 June 2008 (Monday) • Class Website Troubles… Radio spectrum of cold gas cloud X-ray spectrum of hot gas From exploding star Stellar Classification by Spectra The Doppler Shift • Can determine chemical composition of object by emission and absorption spectra, but how? – Compare observed lines with pure lines measured in laboratory • “line catalog” – But any motion of the object will change the observed wavelengths of emission and absorption lines: • Analogy – Firetruck approaches high pitch • Sound waves “pile up” in front of firetruck, moving toward you… – Firetruck recedes low pitch • Sound waves “stretch out” behind firetruck, moving away from you… • Exact same thing can happen with light waves • If atom moves toward you when it emits light: – Wavelength decreases: “blueshifted” • If atom moves away from you when it emits light: – Wavelength increases: “redshifted” Doppler Shift • Physics can get you in trouble with the law… – Photoradar speed-traps use the Doppler effect to measure car speeds Atmospheric Absorption • Gases in Earth’s atmosphere (N2, O2, Ar, CO2) absorb light from distant sources – Sunlight – Astronomical sources • “Atmospheric Window” – The reason our eyes are sensitive to visible light is because it is NOT easily absorbed by the Earth’s atmosphere – This is also the reason why we need space-based telescopes to observe in the IR, UV, X-ray regions of the EM spectrum NEXT TIME • Telescopes – How do we capture all these light waves?