* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Psychopharmacology wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Drug design wikipedia , lookup
Pharmacognosy wikipedia , lookup
Discovery and development of cephalosporins wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Dydrogesterone wikipedia , lookup
Drug interaction wikipedia , lookup
Theralizumab wikipedia , lookup
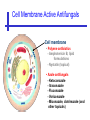
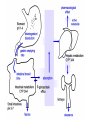
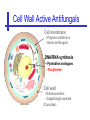
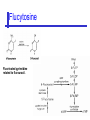
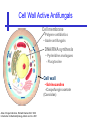
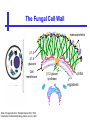
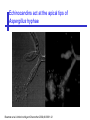
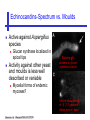
An Introduction to Anti-fungal Pharmacology The following slides were generously supplied by Professor Russell E. Lewis, Pharm.D., BCPS University of Houston College of Pharmacy, University of Texas M.D. Anderson Cancer Center. With lecture notes written by Hannah Woodcock and Jenny Bartholomew, University of Manchester, UK. Types of fungal infections - Mycoses Superficial mycoses Affect the skin, hair and nails Subcutaneous mycoses (tropical) Affect the muscle and connective tissue immediately below the skin Systemic (invasive) mycoses Involve the internal organs Primary vs. opportunistic Allergic mycoses Affect lungs or sinuses Patients may have chronic asthma, cystic fibrosis or sinusitis There is some overlap between these groups What are the targets for antifungal therapy? Cell membrane Fungi use principally ergosterol instead of cholesterol DNA Synthesis Some compounds may be selectively activated by fungi, arresting DNA synthesis. Cell Wall Unlike mammalian cells, fungi have a cell wall Atlas of fungal Infections, Richard Diamond Ed. 1999 Introduction to Medical Mycology. Merck and Co. 2001 Cell Membrane Active Antifungals Cell membrane • Polyene antibiotics - Amphotericin B, lipid formulations - Nystatin (topical) • Azole antifungals - Ketoconazole - Itraconazole - Fluconazole - Voriconazole - Miconazole, clotrimazole (and other topicals) Azole Antifungals for Systemic Infections Ketoconazole (Nizoril) Itraconazole (Sporanox) Fluconazole (Diflucan) Voriconazole (Vfend) Fluconazole Ketoconazole Imidazole Triazoles “2nd generation triazole” Azoles - Mechanism In fungi, the cytochrome P450enzyme lanosterol 14-a demethylase is responsible for the conversion of lanosterol to ergosterol Azoles bind to lanosterol 14ademethylase inhibiting the production of ergosterol Some cross-reactivity is seen with mammalian cytochrome p450 enzymes Drug Interactions Impairment of steroidneogenesis (ketoconazole, itraconazole) Effect of azoles on C. albicans Before exposure After exposure Azoles - Pharmacodynamics Concentration-independent fungistatic agents Dosage escalation may be necessary when faced with more resistant fungal species (e.g. Candida glabrata) Goal of dosing is to maintain AUC:MIC >50 i.e. maintain concentrations 1-2 x MIC for the entire dosing interval Ketoconazole Spectrum: yeasts and moulds - poor absorption limits its role for severe infections, generally used in mucosal infections only Pharmacokinetics Variable oral absorption, dependent on pH (often given with cola or fruit juice) T1/2 7-10 hours Protein binding > 99% Hepatic, bile and kidney elimination Ketoconazole - Adverse effects Adverse effects N&V, worse with higher doses (800 mg/day) Hepatoxicity (2-8%), increase in transaminases, hepatitis Dose related inhibition of CYP P450 responsible for testosterone synthesis Gynecomastia, oligosperma, decreased libido Dose-related inhibition of CYP P450 responsible for adrenal cortisol synthesis Ketoconazole - Drug Interactions Potent inhibitor of cytochrome P450 3A4 Rifampin and phenytoin decrease ketoconazole levels Ketoconazole increases cyclosporin, warfarin, astemizole, corticosteroid, and theophylline levels Many of these drug interactions are severe Drugs that increase gastric pH will decrease blood levels of ketoconazole Antacids, omeprazole, H2 blockers Ketoconazole - Dose Serious infections 800 mg/day PO Other: 200-400 mg/day PO Cost $2.50 per 200 mg tablet Fluconazole Advantages Well tolerated IV/PO formulations Favorable pharmacokinetics Disadvantages Fungistatic Resistance is increasing Narrow spectrum (Drug interactions) Fluconazole - spectrum Good activity against C. albicans and Cryptococcus neoformans Non-albicans Candida species more likely to exhibit primary resistance Always resistant C. krusei > Sometimes resistant C. glabrata > C. parapsilosis C. tropicalis C. kefyr Fluconazole - resistance Primary resistance (seen in severely ill or immunocompromised patients) Selection of resistant species or subpopulations Replacement with more resistant strain Secondary resistance (seen in patients with AIDS who experienced recurrent orophayrngeal candidiasis and received longterm fluconazole therapy) Genetic mutation Upregulation of efflux pumps Mechanisms of antifungal resistance Target enzyme modification Ergosterol biosynthetic pathway Efflux pumps Drug import White TC, Marr KA, Bowden RA. Clin Microbiol Review 1998;11:382-402 Fluconazole - What is not covered Candida krusei +/- Candida glabrata Aspergillus species and other moulds Fluconazole - Pharmacokinetics Available as both IV and PO Bioavailibility > 90% Linear pharmacokinetics t 1/2 = ~24 hours Cmax (400 mg IV) = 20 µg/ml (steady state) Protein binding < 12% Vd 0.85 L/kg (widely distributed) >90% excreted unchanged through the kidney Fluconazole - adverse effects/monitoring N&V, rash: More likely with high doses and in AIDS patients Asymptomatic increase in LFTs (7%) Drug interactions: May increase phenytoin, cyclosporin, rifabutin, warfarin, and zidovudine concentrations Rifampin reduced fluconazole levels to half (even though FLU is not a major substrate) Fluconazole - Dosing Mucosal candidiasis Systemic fungal infections 100-200 mg/day (150 mg tablet vulvovaginal candidiasis) 400-800 mg q24h > 800 mg q24h in unstable patient, S-DD isolate, or if non-albicans spp. (except C. krusei) Maintenance for cryptococcal meningitis 400 mg q24h Key Biopharmaceutical Characteristics of the Triazole Antifungals Fluconazole Itraconazole Spectrum vs. Candida and Aspergillus C. albicans, C. tropicalis +/C. glabrata No Aspergillus Similar Candida coverage as fluconazole, + Aspergillus Broad, includes most Candida spp., Aspergillus, Fusarium sp. Not Zygomycoses Oral formulation (% bioavailibility) Tablet (>90%) Capsule (6-25%) Solution (20-60%) Tablet (>90%) Intravenous formulation Available, no solubilizer Available, cyclodextrin Available, cyclodextrin Clearance Renal (80%) Hepatic 3A4 Hepatic 2C19, 3A4 Serum half life (hr) 24 24-30 6-24 CSF penetration Excellent Poor Excellent CYP 3A4 inhibition Weak Strong Moderate-Strong Adverse effects N&V, hepatic N&V, diarrhea (solution), hepatic, CHF N&V, visual disturbances, hepatic, rash R.E. Lewis 2002. Exp Opin Pharmacother 3:1039-57. Voriconazole Itraconazole Solution - Side Effects Taste disturbances Nausea and vomiting Osmotic diarrhea (especially at doses > 400 mg/day) Long-term compliance often difficult Voriconazole - Side Effects Visual disturbances (~ 30%) Decreased vision, photophobia, altered color perception and ocular discomfort IV > oral No evidence of structural damage to retina Reversible alterations in function of retinal rods and cones Testing 2 weeks after the end of treatment demonstrates a return to normal function Long term effects?..caution against night-time driving Effects may be intensified by hallucinations (2-5%) Amphotericin B Polyene antibiotic Fermentation product of Streptomyces nodusus Binds sterols in fungal cell membrane Creates transmembrane channel and electrolyte leakage. Active against most fungi except Aspergillus terreus, Scedosporium spp. Lipid Amphotericin B Formulations Abelcet ® ABLC Ribbon-like particles Carrier lipids: DMPC, DMPG Particle size (µm): 1.611 Amphotec ® ABCD Disk-like particles Carrier lipids: Cholesteryl sulfate Particle size (µm): 0.120.14 Ambisome ® L-AMB Unilaminar liposome Carrier lipids: HSPC, DSPG, cholesterol Particle size (µm) : 0.08 DMPC-Dimyristoyl phospitidylcholine HSPC-Hydrogenated soy phosphatidylcholine DMPG- Dimyristoyl phospitidylcglycerol DSPG-Distearoyl phosphitidylcholine Amphotericin B Classic amphotericin B deoxycholate (Fungizone™) formulation: serious toxic side effects. Less toxic preparations: 1) Liposomal amphotericin B 2) Amphotericin B colloidal dispersion 3) Amphotericin B lipid complex Amphotericin B - Pharmacokinetics Absorption from the GI tract is negligible High Oral solution sometimes used to decontaminate gut; few side effects Only reliable method of administration is IV Selective distribution into deep tissue sites, with slow release of drug Low kidney > liver > spleen > lung > heart > skeletal muscle > brain > bone > CSF > eye Amphotericin B - Metabolic elimination Metabolic fate is unknown, drug accumulates in tissues and then is slowly released Drug levels can be measured in the kidney, liver, and spleen up to 1 year after receiving drug Dosages of amphotericin B are generally not altered due to decreased elimination of the drug in kidney dysfunction Hemodialysis does not alter serum drug concentrations except in hyperlipidemic patients Amphotericin B - Elimination Inverse correlation between patient age and elimination of AmB, Age, elimination, side effects Paediatric patients often tolerate amphotericin B better than adults Amphotericin B - Nephrotoxicity Most significant delayed toxicity Renovascular and tubular mechanisms Vascular-decrease in renal blood flow leading to drop in GFR, azotemia Tubular-distal tubular ischemia, wasting of potassium, sodium, and magnesium Enhanced in patients who are volume depleted or who are on concomitant nephrotoxic agents Amphotericin B - Manoeuvers employed to blunt nephrotoxicity… Sodium loading-> blunt the vasoconstriction and tubular-glomerular feedback Administration of 500 ml -1000 ml of NaCl before and after amphotericin B infusion Amphotericin B - Drug Interactions Enhanced nephrotoxicity Nephrotoxic drugs Cyclosporine, aminoglycosides, foscarnet, pentamidine Antineoplastic agents Cisplatin, nitrogen mustards Amphotericin B - Clinical Uses The drug of choice for: Cryptococcal meningitis Mucormycosis (zygomycosis) Invasive fungal infection, not responding to other therapy Amphotericin B - Dosing and Administration “Test dose” 1.0 mg in 25-100ml 5% dextrose infused over 10 minutes used to evaluate possibility of anaphylactic reaction No longer recommended, current product has fewer impurities Current recommendation- Start with ~30% of target dose, infuse for 15 minutes, stop infusion, and monitor patient for adverse effects before resuming infusion Rapidly escalate to full dosages within 48-72 hours Delay in giving full dose = worse clinical outcome Cell Wall Active Antifungals Cell membrane • Polyene antibiotics • Azole antifungals DNA/RNA synthesis • Pyrimidine analogues - Flucytosine Cell wall • Echinocandins -Caspofungin acetate (Cancidas) Flucytosine Fluorinated pyrimidine related to flurouracil. Flucytosine Restricted spectrum of activity. Acquired Resistance. > result of monotherapy > rapid onset Due to: 1) Decreased uptake (permease activity) 2) Altered 5-FC metabolism (cytosine deaminase or UMP pyrophosphorylase activity) Flucytosine - pharmacokinetics Oral absorption complete Plasma half-life 3-6 hrs Volume of distribution 0.7-1l/kg (low) Plasma protein binding ~12% Flucytosine - side effects Infrequent – include D&V, alterations in liver function tests and blood disorders. Blood concs need monitoring when used in conjunction with Amphotericin B. Flucytosine – Clinical uses Monotherapy : now limited Candidiasis Cryptococcosis ?Aspergillosis } In combination with amphotericin B or fluconazole. Cell Wall Active Antifungals Cell membrane • Polyene antibiotics • Azole antifungals DNA/RNA synthesis • Pyrimidine analogues - Flucytosine Cell wall • Echinocandins -Caspofungin acetate (Cancidas) Atlas of fungal Infections, Richard Diamond Ed. 1999 Introduction to Medical Mycology. Merck and Co. 2001 The Fungal Cell Wall mannoproteins b1,3 b1,6 glucans Cell membrane b1,3 glucan synthase chitin ergosterol Atlas of fungal Infections, Richard Diamond Ed. 1999 Introduction to Medical Mycology. Merck and Co. 2001 Echinocandins - Pharmacology Cyclic lipopeptide antibiotics that interfere with fungal cell wall synthesis by inhibition of ß-(1,3) Dglucan synthase Loss of cell wall glucan results in osmotic fragility Spectrum: HO H O H HO NH H NH O N H HO O H H H3 C OH NH O CH3 H H H H2N NH H O N O HO H OH H OH NH H OH H H O OH Candida species including nonalbicans isolates resistant to fluconazole Aspergillus spp. but not activity against other moulds (Fusarium, Zygomycosis) No coverage of Cryptococcus neoformans Echinocandins - spectrum Highly active Very active Some activity Candida albicans, Candida glabrata, Candida tropicalis, Candida krusei Candida kefyr Pneumocystis carinii Candida parapsilosis Candida gulliermondii Aspergillus fumigatus Aspergillus flavus Aspergillus terreus Candida lusitaniae Coccidioides immitis Blastomyces dermatididis Scedosporium species Paecilomyces variotii Histoplasma capsulatum Low MIC ,with fungicidal activity and good in-vivo activity. Low MIC, but without fungicidal activity in most instances. Detectable activity, which might have therapeutic potential for man (in some cases in combination with other Echinocandins act at the apical tips of Aspergillus hyphae DiBAC Bowman et al. Antimicrob Agent Chemother 2002;46:3001-12 Echinocandins-Spectrum vs. Moulds Active against Aspergillus species Glucan synthase localized in apical tips Activity against other yeast and moulds is less well described or variable Staining with antisera to glucan synthase subunit (Fks1p) Mycelial forms of endemic mycoses? Aniline blue staining of β (1-3) glucans – stains only at apex Beauvais et al. J. Bacteriol 2001;183:2273-79 Caspofungin - Pharmacokinetics Absorption < 2% Distribution (Vd) 9.67 L Protein binding 97% albumin Major metabolic pathway Peptide hydrolysis, slow N-acetylation t 1/2 ß 9-11 hours CNS penetration Probably poor Dosage adjustment Moderate-severe hepatic dysfunction Drug-Drug interactions Significant interactions CSA? FK506, mycophenolate? Inducers of 3A4? Caspofungin acetate IV only Indication: Invasive candidiasis Invasive aspergillosis refractory to other therapies Dosage and administration 70 mg day 1, followed by 50 mg daily Increase to 70 mg per day in non-responders Decrease to 35 mg per day in moderate-severe hepatic dysfunction (Child-Pugh 7-9) Antiviral Drug Products Advisory Committee, January 10, 2001- www.FDA.gov Caspofungin - Adverse effects Most common AEs are infusion related: Intravenous site irritation (15-20%) Mild to moderate infusion-related AE including fever, headache, flushing, erythema, rash (5-20%) Symptoms consistent with histamine release (2%) Most AEs were mild and did not require treatment discontinuation Most common laboratory AE Asymptomatic elevation of serum transaminases (10-15%) Clinical experience to date suggests that these drugs are extremely well-tolerated Antiviral Drug Products Advisory Committee, January 10, 2001- www.FDA.gov Drug Dosage Polyenes Amphotericin B deoxycholate Lipo-AMB (AmBisome) ABLC (Abelcet) Amphocil AWP Cost/day for 70kg Patient 1 mg/kg/day IV 3 mg/kg/day IV 5 mg/kg/day IV 3 mg/kg/day IV £7 £554 £246 £380 Triazoles Fluconazole Itraconazole Voriconazole 400/800 mg IV 400 mg IV 4 mg/kg IV £56/£112 £72 £80 Echinocandins Caspofungin 50 mg IV x 1 day, £334 (Medical Letter 2002;44:63-65; Lancet 2003;362:1142-1151)