* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download New Fungal Drugs
Survey
Document related concepts
Transcript
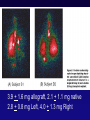
Amphotericinor Echinocandin-Based Antifungal Prophylaxis Approaches Jo-Anne Young, MD, FACP, FIDSA Disclosures Clinical Trials funding support (antifungal agents): Pfizer, Astellas, Merck, Schering-Plough Clinical Trials funding support (outside of mycology): Glaxo, ViroPharma, Advanced Biologics, Adamas Not a Consultant Not on a Speaker’s Bureau Objectives Amphotericin as antifungal prophylaxis Merits, Problems Echinocandins as antifungal prophylaxis Merits, Problems Undefined areas in practice Amphotericin routes Oral ICU Selective digestive tract decontamination Radiotherapy lung cancer patients Nebulization or nasal spray Lung transplant HSCT IV Standard care SDD consisted of 4 days of intravenous cefotaxime and topical application of tobramycin, colistin, and amphotericin B in the oropharynx and stomach. SOD consisted of oropharyngeal application only of the same antibiotics. Nonrandomized study of lung cancer consecutive patients 20 patients 67 Gy (range 61-80 Gy) AmB QID from day 8 to the end of radiotherapy Trend toward higher esophageal volumes Older Worse median Karnofsky Index More often received induction chemotherapy Start of symptoms day 21 (median, range 14-44) 5 patients developed esophagitis grade 1 20 patients 60 Gy (range 51-67.5 Gy) control group Start of symptoms day 18 (median, range 10-32) 14 patients showed esophagitis grade 1 and 2 patients grade 2 (p < 0.05). Nasal Bottle design to prevent aspiration of nasal secretions back into the bottle Each nostril 5x/day Compliance a problem Irritating to nasal mucosa: tolerance a problem Reduction in surveillance CFUs, but not in invasive disease Bronchial Anastamosis Courtesy of Jordan Dunitz, MD 6 single and 6 double lung recipients One 7-ml (35 mg) nebulized dose labeled with Technetium Selected system: AeroEclipse nebulizer & DeVilbiss compressor 3.9 + 1.6 mg allograft, 2.1 + 1.1 mg native 2.8 + 0.8 mg Left, 4.0 + 1.3 mg Right 271 patients 407 neutropenic episodes. Some adverse effects, but none serious, in the liposomal amphotericin B group were reported, most frequently coughing (16 patients vs. 1 patient; P=.002). Prophylactic inhalation of liposomal amphotericin B significantly reduced the incidence of IPA. Intent-to-treat analysis 18 of 132 patients in the placebo group developed IPA 6 of 139 patients in the liposomal amphotericin B group odds ratio, 0.26; 95% CI, 0.09-0.72; P=.005 On-treatment analysis 13 of 97 patients receiving placebo developed IPA 2 of 91 receiving liposomal amphotericin B odds ratio, 0.14; 95% CI, 0.02-0.66; P=.007 Nebulization: merits Directed delivery to the lungs, good distribution throughout lung airways Achieves high local concentration Half-life in lung of 4.8 days Not associated with a decline in PFTs Avoids undesirable systemic effects and drug interactions Lipid formulations Penetrate the lung better Have a longer half-life Administer at long intervals No demonstrated resistance Nebulization: problems Some increased risk of Transient cough Nausea Aftertaste Deoxycholate detergent may have adverse effects on surfactant Breakthrough invasive disease Pulmonary Cerebral Nebulization: undefined areas Many type of nebulizers Only a few dosages studied No data regarding Long term efficacy Repeated use efficacy Comparison to systemic antifungals Synergy with systemic antifungals Lack of standardization of administration procedures and doses These are not treatment data IV Potential systemic toxicity Amphotericin accumulates in the reticuloendothelial system Even a single dose may proved tissue depots for prophylaxis in at-risk pts such as liver transplant Intermittent high dosing of lipid ampho may be an option to daily azoles or candins Enrollment was discontinued in the SCT group as recommended by the independent data review committee in accordance with the 10% limit of AEs (CTC grade 3-4) fixed by the protocol. Yeasts Molds Randomized, double-blind Phase III study 72 centers in the US and Canada Patient population: HCT candidates 6 months of age Autologous HCT for heme malignancies only CID 2004;39:1407-16 (van Burik et al.) Treatment success Treatment difference P=0.03 Micafungin Fluconazole 340 / 425 336 / 457 (80%) (73.5%) +6.5% (95% CI, 0.9% to 12%) Assess non-inferiority of micafungin to fluconazole over 10% Treatment success Absence of suspected, proven, or probable invasive fungal infection through the end of prophylaxis period Absence of a proven or probable invasive fungal infection through the end of the 4-week post-treatment period Proportion of Patients with Treatment Success Time to Treatment Failure 1 0.9 0.8 0.7 0.6 0.5 Micafungin (N=425) Fluconazole (N=457) 0.4 0.3 P-Value (2 tailed) = 0.025 0.2 0.1 0 0 10 20 30 40 50 60 70 Days Since First Dose of Study Drug P=0.07 Micafungin compared with Fluconazole Micafungin Fluconazole Breakthrough fungal infection 7/425 11/457 (1.6%) (2.4%) Aspergillus* 1 7 Proven 0 4 Probable 1 3 Candida 4 2 Fusarium 1 2 Zygomycetes 1 0 Death 18/425 26 / 457 (4.2%) (5.7%) Death due to FI 1 (Zygomycetes) 2 (Pulmonary aspergillosis) HSCT candidates 6 months of age Micafungin Pediatric <16 yrs 69% (27/39) Adult 16-64 yrs 81% (313/386) Adult > 64 yrs 97% (32/33) Fluconazole 53% (24/45) 76% (312/412) 70% (16/23) High dose micafungin Adult patients Micafungin 150 mg (n = 52) or Fluconazole 400 mg (n = 52) Success 94 vs. 88% Empirical antifungal therapy (P = 0.06) 2/50 (4.0%) micafungin 6/50 (12.0%) fluconazole arm Int J Hematol. 2008 Dec;88(5):588-95 Liver transplant Open-label trial of 71 adult liver transplant recipients Caspofungin for at least 21 days 2 IFI: Mucor and Candida albicans surgical wound infections 6 discontinued: drug-related altered liver function 8 patients died, 6 during caspofungin administration and 2 during follow-up period, but none were attributed to IFI or caspofungin toxicity Transplantation 2009 Feb 15;87(3):424-35 Echinocandins: undefined areas Limited amount of published data: Randomized trials Observational cohorts Specific patient populations By disease By age Can trials with one drug be extrapolated to other drugs Dosage Summary Amphotericin as antifungal prophylaxis Merits, Problems Echinocandins as antifungal prophylaxis Merits, Problems Undefined areas in practice