* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Bohr Model of the Atom
Survey
Document related concepts
Transcript
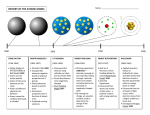
The Bohr Model of the Atom Atomic Model Revisions • Dalton’s model of the atom assumed the atom to be an indestructible mass. This explained the nature of chemical reactions to a large degree. • But the idea of indivisible atoms was shattered by Thomson and Rutherford. • Thomson came up with his own theory, called the plum pudding model, which assumed that electrons were imbedded in a positively charged matrix. • This model addressed some, but not all, of the electrical properties of atoms. Further Revisions: The Rutherford Model • Rutherford’s model of the atom composed of a positively charged nucleus surrounded by electrons explained a few properties of atoms, but not all. • In particular, it did not explain why many atoms emit light of specific frequencies when heated. • Rutherford also could not explain why electrons did not collapse into the nucleus. Big Problem • Neither Rutherford nor Thomson’s models could explain why the electrons of elements and compounds emit specific wavelengths of light, rather than a complete spectrum. • This “bar code” is unique for hydrogen. The lines pictured above are known as the “Balmer series.” • Hydrogen has several other series of lines that are outside the visible range. • For most of the 19th century, spectroscopists puzzled over these lines for a wide variety of substances, with little to show for it. Emission Spectra of Elements • Each element gives off a unique selection of wavelengths. • Only the visible spectrum is shown here! There are more lines than these. Lab 4 Emission Spectra of Elements • Look at Ne and Ar spectrum tubes with your spectroscope. Draw one set of emission spectra in the boxes below. Neon Wavelength Argon Wavelength Argon and Neon Spectra • On the diagrams on the following page, draw a thick line with an appropriate colored pencil through each of the major peaks that correspond to the lines you see with the spectroscope. • Estimate the wavelengths, in nanometers, that correspond to at least some of the lines that you see using your spectrograph. Neon Spectrum Argon Spectrum Questions • How do your drawings of the emission spectra compare to the emission spectra on the following page? • Do you have the same lines, or are some missing? • Is the spacing between the lines similar, or different? Identifying Elements Using Spectra • Using the data on the back, how could someone distinguish between samples of Ne and Ar gases without observing them directly in a cathode ray tube? Atomic Theory Test Multiple Choice Breakdown • • • • • • • • Rutherford model: Nuclear structure: What is the atomic number? What is the mass number? How many neutrons? How many electrons? Atomic mass definition Atomic mass calculation 4 questions 4 questions 8 questions 2 questions 3 questions 2 questions 3 questions 3 questions Modern atomic theory begins with Niels Bohr (1885-1962). In 1913, Niels Bohr explained why the lines in the hydrogen spectrum are arranged the way they are. His explanation places electrons in stable orbits which he called "stationary states." These states violated well-known laws of science. Bohr saw spectrum lines as the energy difference resulting from the movement of an electron from a higher-energy stationary state to a lower-energy one. • Bohr proposed that electrons are arranged in specific circular paths around the nucleus – a planetary model. • His major contribution was to propose that electrons in a particular orbit have a fixed energy, thus avoiding falling into the nucleus. • The energy level of an electron is the region around the nucleus where the electron is likely to be moving. • According to Bohr, electrons cannot exist between the energy levels – they have to “jump” from one level to another. • To do this, they must gain or lose a specific amount of energy. • A quantum of energy is the amount of energy required to move an electron from its present energy level to the next higher one. • The energies of electrons are quantized. The term “quantum leap” comes from this idea. • The quanta gained or lost by every electron can differ. • Energy levels in an atom are not equally spaced. • As the distance from the nucleus increases, the energy levels are more closely spaced. • The spectrum of hydrogen contains three major sets of spectral lines: • The Lyman series (ultraviolet) • The Balmer series (visible) • The Paschen series (infrared) • When the electron is in its lowest energy state, n=1, the “ground state.” • The electron absorbs a quanta of energy, raising it to an excited state where n=2,3,..6. • You can’t “see” absorption, only emission • The absorption spectra have missing lines More on the Bohr Model • The same quanta are emitted when the electron drops from the excited state to the ground state. • Only electrons that “fall” from higher to lower energy states emit light. • Bohr accounted for the hydrogen spectrum by recognizing the lines represented those drops into lower energy states. Hydrogen Spectrum • The Lyman series (ultraviolet) n>1 drops to n=1 • The Balmer series (visible) n>2 drops to n=2 • The Paschen series (infrared) n> 3 drops to n=3 • Note that other series for n>3 also exist. There is a limit to each series because really excited electrons escape the hold of the nucleus. • Bohr’s theory did not explain the spectra of any other element, or any compound. Bohr Model Limitations • Bohr’s notion of energy levels of electrons resulted in a complete explanation for a single element – hydrogen. • Why was he able to explain only hydrogen? It only has one electron. He could also explain a He+ ion or Li2+ ion, which also have only one electron. • When multiple electrons are involved, they interact with each other in complicated ways – called the “three-body problem.”