* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Tanabe-Sugano Diagram Jahn-Teller Effect
Survey
Document related concepts
Transcript
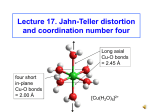
4/8/2012 Tanabe-Sugano Diagram Jahn-Teller Effect In order to accurately interpret the electronic spectra of transition metal complexes, a series of diagrams have been created. These diagrams are used to assign transitions (initial energy state and final energy state) to peaks observed in the spectra, and to calculate the value of ∆o. Tanabe-Sugano diagrams have the lowest energy state (the ground state) plotted along the horizontal axis. The energy of excited states can then be readily compared to the ground state. Yuniar Ponco Prananto Tanabe-Sugano diagrams are used in coordination chemistry to predict absorptions in the UV and visible electromagnetic spectrum of coordination compounds. The results from a Tanabe-Sugano diagram analysis of a metal complex can also be compared to experimental spectroscopic data. They are qualitatively useful and can be used to approximate the value of 10Dq, the ligand field splitting energy. Tanabe-Sugano diagrams can be used for both high spin and low spin complexes. Tanabe-Sugano diagrams can also be used to predict the size of the ligand field necessary to cause high-spin to low-spin transitions. In a Tanabe-Sugano diagram, the ground state is used as a constant reference. The energy of the ground state is taken to be zero for all field strengths, and the energies of all other terms and their components are plotted with respect to the ground term. The x-axis of a Tanabe-Sugano diagram is expressed in terms of the ligand field splitting parameter, Dq, or Δ, divided by the Racah parameter B. The y-axis is in terms of energy, E, also scaled by B. Three Racah parameters exist, A, B, and C, which describe various aspects of interelectronic repulsion. A is an average total interelectron repulsion. A is constant among d-electron configuration, and it is not necessary for calculating relative energies, hence its absence from Tanabe and Sugano's studies of complex ions. B and C correspond with individual delectron repulsions. C is necessary only in certain cases. B is the most important of Racah's parameters in this case. One line corresponds to each electronic state. The bending of certain lines is due to configuration interactions of the excited states. 1 4/8/2012 Hund’s Rules Although electronic transitions are only "allowed" if the spin multiplicity remains the same (i.e. electrons do not change from spin up to spin down or vice versa when moving from one energy level to another), energy levels for "spinforbidden" electronic states are included in the diagrams. Each state is given its symmetry label (e.g. A1g, T2g, etc.), but "g" and "u" subscripts are usually left off because it is understood that all the states are gerade. Labels for each state are usually written on the right side of the table, though for more complicated diagrams (e.g. d6) labels may be written in other locations for clarity. Term symbols (e.g. 3P, 1S, etc.) for a specific dn free ion are listed, in order of increasing energy, on the y-axis of the diagram. The relative order of energies is determined using Hund's rules. In atomic physics, Hund's rules refer to a set of rules formulated by German physicist Friedrich Hund around 1927, which are used to determine the term symbol that corresponds to the ground state of a multi-electron atom. In chemistry, rule one is especially important and is often referred to as simply Hund's rules. The three rules are: For a given electron configuration, the term with maximum multiplicity has the lowest energy. Since multiplicity is 2S+1 equal to , this is also the term with maximum S. S is the spin angular momentum. For a given multiplicity, the term with the largest value of L has the lowest energy, where L is the orbital angular momentum. For a given term, in an atom with outermost sub-shell half-filled or less, the level with the lowest value of J lies lowest in energy. If the outermost shell is more than half-filled, the level with highest value of J is lowest in energy. J is the total angular momentum, J = L + S. These rules specify in a simple way how the usual energy interactions dictate the ground state term. The rules assume that the repulsion between the outer electrons is very much greater than the spin-orbit interaction which is in turn stronger than any other remaining interactions. This is referred to as the LS coupling regime. Full shells and sub-shells do not contribute to the quantum numbers for total S, the total spin angular momentum and for L, the total orbital angular momentum. It can be shown that for full orbitals and sub-orbitals both the residual electrostatic term (repulsion between electrons) and the spin-orbit interaction can only shift all the energy levels together. Thus when determining the ordering of energy levels in general only the outer valence electrons need to be considered. Splitting of Term Symbols from Spherical to Octahedral Symmetry Term S P D F G H I Degeneracy States in an octahedral field 1 A1g 3 T1g 5 Eg + T2g 7 A2g + T1g + T2g 9 A1g + Eg + T1g + T2g 11 Eg + T1g + T1g + T2g 13 A1g + A2g + Eg + T1g + T2g + T2g 2 4/8/2012 Certain Tanabe-Sugano diagrams (d4, d5, d6, and d7) also have a vertical line drawn at a specific Dq/B value, which corresponds with a discontinuity in the slopes of the excited states' energy levels. This pucker in the lines occurs when the spin pairing energy, P, is equal to the ligand field splitting energy, Dq. Complexes to the left of this line (lower Dq/B values) are high-spin, while complexes to the right (higher Dq/B values) are low-spin. There is no low-spin or high-spin designation for d2, d3, or d8. d4 --electron configurations-- d5 d2 --electron configurations-- d3 d6 --electron configurations-- d7 3 4/8/2012 Unnecessary diagrams: d1, d9 and d10 d1 There is no electron repulsion in a d1 complex, and the single electron resides in the t2g orbital ground state. A d1 octahedral metal complex, such as [Ti(H2O)6]3+, shows a single absorption band in a UV-vis experiment. The term symbol for d1 is 2D, which splits into the 2T2g and 2Eg states. The t2g orbital set holds the single electron and has a 2T2g state energy of -4Dq. When that electron is promoted to an eg orbital, it is excited to the 2Eg state energy, +6Dq. This is in accordance with the single absorption band in a UV-vis experiment. Thus, this simple transition from 2T2 to 2Eg does not require a Tanabe-Sugano diagram. d8 electron configurations • d9 Similar to d1 metal complexes, d9 octahedral metal complexes have 2D spectral term. The transition is from the (t2g)6(eg)3 configuration (2Eg state) to the (t2g)5(eg)4 configuration (2T2g state). This could also be described as a positive "hole" that moves from the eg to the t2g orbital set. The sign of Dq is opposite that for d1, with a 2Eg ground state and a 2T2g excited state. Like the d1 case, d9 octahedral complexes do not require the Tanabe-Sugano diagram to predict their absorption spectra. Applications as a qualitative tool In a centrosymmetric ligand field, such as in octahedral complexes of transition metals, the arrangement of electrons in the d-orbital is not only limited by electron repulsion energy, but it is also related to the splitting of the orbitals due to the ligand field. This leads to many more electron configuration states than is the case for the free ion. The relative energy of the repulsion energy and splitting energy defines the high-spin and low-spin states. Considering both weak and strong ligand fields, a Tanabe-Sugano diagram shows the energy splitting of the spectral terms with the increase of the ligand field strength. It is possible for us to understand how the energy of the different configuration states is distributed at certain ligand strengths. The restriction of the spin selection rule makes it is even easier to predict the possible transitions and their relative intensity. • d10 There are no d-d electron transitions in d10 metal complexes because the d orbitals are completely filled. Thus, UV-vis absorption bands are not observed and a Tanabe-Sugano diagram does not exist. Although they are qualitative, Tanabe-Sugano diagrams are very useful tools for analyzing UV-vis spectra: they are used to assign bands and calculate Dq values for ligand field splitting. 4 4/8/2012 Note: Interpretation of Spectra – d3 and d8 Tanabe-Sugano diagrams are utilized in determining electron placements for high spin and low spin metal complexes. However, they are limited in that they have only qualitative significance. Even so, Tanabe-Sugano diagrams are useful in interpreting UV-vis spectra and determining the value of 10Dq. Tetrahedral Tanabe-Sugano diagrams are not commonly found in textbooks because ΔT for tetrahedral complexes is approximately 4/9 of ΔO for an octahedral complex. The consequence of the magnitude of ΔT results in the tetrahedral complexes being high spin. Orgel diagrams are best used for the treatment of tetrahedral complexes. Interpretation of Spectra – d3 and d8 ν1 The Tanabe-Sugano diagram can be used to assign transitions to each absorption. Interpretation of Spectra – d3 and d8 ν1 ν1 The first peak is due to the 4A2g(F) 4T2g(F) transition and has an energy equal to ∆o. ν2 ν2 The second peak is due to the 4A2g(F) 4T1g(F) transition. 5 4/8/2012 Interpretation of Spectra – d3 and Interpretation of Spectra – d5 (high spin) d8 ν3 ν1 ν2 ν3 Mn2+ compounds are white to pale pink in color. The third peak is due to the 4A2g(F) 4T1g(P) transition. There are no spin allowed transitions for d5 high spin configurations. Extinction coefficients are very low, though the selection rule is relaxed by spin-orbit coupling. References Jahn-Teller Distortion Racah, Giulio (1942). "Theory of complex spectra II". Physical Review 62: 438–462. Tanabe, Yukito; Sugano, Satoru (1954). "On the absorption spectra of complex ions I". Journal of the Physical Society of Japan 9 (5): 753–766. 3. Tanabe, Yukito; Sugano, Satoru (1954). "On the absorption spectra of complex ions II". Journal of the Physical Society of Japan 9 (5): 766–779. 4. Tanabe, Yukito; Sugano, Satoru (1956). "On the absorption spectra of complex ions III". Journal of the Physical Society of Japan 11 (8): 864–877. 5. Atkins, Peter; Overton, Tina; Rourke, Jonathan; Weller, Mark; Armstrong, Fraser; Salvador, Paul; Hagerman, Michael; Spiro, Thomas et al (2006). Shriver & Atkins Inorganic Chemistry (4th ed.). New York: W.H. Freeman and Company. pp. 478–483. 6. Douglas, Bodie; McDaniel, Darl; Alexander, John (1994). Concepts and Models of Inorganic Chemistry (3rd ed.). New York: John Wiley & Sons. pp. 442–458. 7. Cotton, F. Albert; Wilkinson, Geoffrey; Gaus, Paul L. (1995). Basic Inorganic Chemistry (3rd d.). New York: John Wiley & Sons. pp. 530–537. 8. Bertolucci, Daniel C. (1978). Symmetry and Spectroscopy: An Introduction to Vibrational and Electronic Spectroscopy. New York: Dover Publications, Inc.. pp. 403–409, 539. 9. Lancashire, Robert John (4–10 June 1999), "Interpretation of the spectra of first-row transition metal complexes", CONFCHEM, ACS Division of Chemical Education 10. Lancashire, Robert John (25 September 2006). "Tanabe-Sugano diagrams via spreadsheets". Retrieved 29 November 2009. 11. Jørgensen, Chr Klixbüll (1954). "Studies of absorption spectra IV: Some new transition group bands of low intensity". Acta Chem. Scand. 8 (9): 1502–1512. 12. Jørgensen, Chr Klixbüll (1954). "Studies of absorption spectra III: Absoprtion Bands as Gaussian Error Curves". Acta Chem. Scand. 8 (9): 1495–1501. 1. 2. Long axial Cu-O bonds = 2.45 Å four short in-plane Cu-O bonds = 2.00 Å [Cu(H2O)6]2+ 6 4/8/2012 The Jahn-Teller Theorem Structural effects of Jahn-Teller distortion The Jahn-Teller (J-T) theorem states that in molecules/ ions that have a degenerate ground-state, the molecule/ion will distort to remove the degeneracy. This is a fancy way of saying that when orbitals in the same level are occupied by different numbers of electrons, this will lead to distortion of the molecule. For us, what is important is that if the two orbitals of the eg level have different numbers of electrons, this will lead to J-T distortion. Cu(II) with its d9 configuration is degenerate and has J-T distortion: High-spin Ni(II) – only one way of filling the eg level – not degenerate, no J-T distortion All six Ni-O bonds equal at 2.05 Å two long axial Cu-O bonds = 2.45 Å Cu(II) – two ways of filling eg level – it is degenerate, and has J-T distortion d9 d8 energy Ni(II) eg t2g eg eg t2g t2g Splitting of the d-subshell by Jahn-Teller distortion The CF view of the splitting of the d-orbitals is that those aligned with the two more distant donor atoms along the z-coordinate experience less repulsion and so drop in energy (dxz, dyz, and dz2), while those closer to the in-plane donor atoms (dxy, dx2-y2) rise in energy. An MO view dx2-y2 of the splitting is that the dx2-y2 in eg particular overlaps energy more strongly with dz2 the ligand donor orbitals, and so is dxy raised in energy. Note t2g that all d-orbitals with Cu(II) in regular octaa ‘z’ in the subscript hedral environment dxz dyz drop in energy. four short in-plane Cu-O bonds = 2.00 Å [Cu(H2O)6]2+ J-T distortion lengthens axial Cu-O’s [Ni(H2O)6]2+ no J-T distortion Structural effects of Jahn-Teller distortion on [Cu(en)2(H2O)2]2+ long axial Cu-O bonds of 2.60 Å water N N Cu N Short in-plane Cu-N bonds of 2.03 Å N ethylenediamine CCD:AZAREY Cu(II) after J-T distortion 7 4/8/2012 Thermodynamic effects of Jahn-Teller distortion: Structural effects of Jahn-Teller distortion on [Cu(en)3]2+ log K1(en) as a function of no of d-electrons Cu(II) 12 N 10 N N Cu logK1(en). Short in-plane Cu-N bonds of 2.07 Å N N doublehumped curve 8 6 Zn2+ 4 Ca2+ 2 N 0 1 CCD:TEDZEI LFSE Mn2+ d2 d3 d4 d5 d6 d7 0 .4Δo .8 1.2 .6 0 .4 .8 d8 2 3 4 5 6 7 8 9 10 11 d-electrons configuration Experimental Evidence of LFSE d1 rising baseline due to ionic contraction 0 long axial Cu-N bonds of 2.70 Å do Extra stabilization due to J-T distortion = CFSE d-electron configurations that lead to Jahn-Teller distortion: d9 1.2 .6 d10 energy 0 eg eg t2g d4 high-spin Cr(II) Mn(III) t2g d7 low-spin Co(II) Ni(III) eg eg t2g t2g d8 low-spin Co(I), Ni(II), Pd(II) Rh(I),Pt(II), Au(III) d9 Cu(II) Ag(II) 8 4/8/2012 The strength of the Jahn-Teller effect is tabulated below: (w=weak, s=strong) Σ eHigh spin Low spin 1 2 3 4 5 6 7 8 9 10 * * * s - w w * * w w - w w - s - s Charge Transfer Spectra * - *There is only 1 possible ground state configuration. - No Jahn-Teller distortion is expected. Yuniar Ponco Prananto Many transition metal complexes exhibit strong chargetransfer absorptions in the UV or visible range. These are much more intense than dd transitions, with extinction coefficients ≥ 50,000 L/mol-cm (as compared to 20 L/molcm for dd transitions). Examples of these intense absorptions can be seen in the permanganate ion, MnO4-. They result from electron transfer between the metal and the ligands. In charge transfer absorptions, electrons from molecular orbitals that reside primarily on the ligands are promoted to molecular orbitals that lie primarily on the metal. This is known as a charge transfer to metal (CTTM) or ligand to metal charge transfer (LMCT). The metal is reduced as a result of the transfer. 9 4/8/2012 LMCT typically occurs in complexes with the metal in a fairly high oxidation state. It is the cause of the intense color of complexes in which the metal, at least formally, has no d electrons (CrO42-, MnO41-). LMCT occurs in the permangate ion, MnO41-. Electrons from the filled p orbitals on the oxygens are promoted to empty orbitals on the manganese. The result is the intense purple color of the complex. MLCT typically occurs in complexes with π acceptor ligands. The empty π* orbitals on the ligands accept electrons from the metal upon absorption of light. The result is oxidation of the metal. Examples of MLCT include iron(III) with acceptor ligands such as CN- or SCN-. The complex absorbs light and oxidizes the iron(III) to iron(IV) state. The metal may be in a low oxidation state (0) with carbon monoxide as the ligand. Many of these complexes are brightly colored, and some appear to exhibit both types of electron transfer. Latihan Soal 3. Jelaskan 4 faktor yang mempengaruhi nilai 10Dq (Δo)! 1. Jelaskan apa yang dimaksud dengan: (a) prinsip keelektronetralan Pauling; (b) CFSE; (c) deret spektrokimia; (d) kompleks medan kuat dan medan lemah; (e) Efek Jahn-Teller! 4. Gambarkan dua orbital molekul dari kompleks [NiCl4]2(Ar Ni = 28) ketika bersifat diamagnetik maupun paramagnetik! 2. Jelaskan kelebihan dan kelemahan teori berikut dalam menjelaskan sifat – sifat senyawa kompleks (a) Teori ikatan valensi; (b) Teori medan kristal; (c) dan Teori orbital molekul! 6. Mengapa pada kompleks Co(II) medan kuat cenderung terjadi distorsi Jahn-Teller, sedangkan pada kompleks Co(II) medan lemah tidak? 5. Jelaskan mengapa konfirgurasi elektron d1, d9, dan d10 tidak memiliki / memerlukan diagram Tanabe-Sugano! 10