* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Ligand Field Theory in the New Millenium: Is there Life after DFT?
Survey
Document related concepts
Transcript
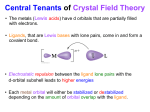
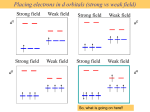
Electronic Effects and Ligand Field Theory Dr Rob Deeth Inorganic Computational Chemistry Group University of Warwick UK Overview • • • • • • • • • Introduction Electronic effects in TM chemistry Classical v. Organometallic compounds Ligand Field Stabilisation Energy d orbitals Spin states and Jahn-Teller effects Generalised ligand field theory Ligand Field Molecular Mechanics DommiMOE Electronic Effects • Geometric preferences • Obvious ones: – Jahn-Teller effect = distorted, especially Cu(II) – four short, two long • Less obvious ones: – Low-spin d8 = planar, especially Pd(II), Pt(II), Rh(I) – Low-spin d6 = octahedral, Co(III) • First row TMs particularly complicated Plasticity • M-L bonds weaker than C-C • Higher coordination numbers • More flexible geometry – angular variations – [CuCl4]2– High spin NiL4 – tetrahedral – Low spin NiL4 – planar – Five coordination – small energy difference between square pyramidal and trigonal bipyramidal Classical v. Organometallic • Werner-type: – Relatively ionic – Electronic effects focussed on d orbitals – IONS • Organometallic – Relatively covalent – More general electronic effects – spndm – Neutral or +-1 • For classical coordination complexes, need to consider d orbitals d orbitals Z Z Z Y Y Y X X dx2-y2 X d2z2-x2-y2 dxz Y X X Z Z Y dxy dyz d Orbital splittings • In octahedral symmetry, the five d orbitals split • Barycentre relative to average d orbital energy eg +3/5 n+ M 10Dq d -2/5 n+ Free M t2g ion n+ M in octehdral crystal field Point charge q = ze ∆oct Ligand Field Stabilisation Energy • Structural preferences and Jahn-Teller instabilities can be traced to LFSE • LFSE d0: 0 d1: -2/5∆oct d2: -4/5∆oct d3: -6/5∆oct d4: -3/5∆oct d5: 0 ∆Hhyd Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Spin States • • • For dn configurations with 2 ≤ n ≤ 8, multiple spin states are possible Spin depends on symmetry and ligands Consider octahedral complexes – Spin state a balance between d orbital splitting and spin pairing energy S=2 4 S = 1/2 S=1 S = 3/2 d 1 d S=1 S = 5/2 2 4 3 d 5 d d d high low high S = 1/2 5 d low S = 3/2 5 d intermediate π Bonding Affects ∆oct • σ-only ligand leaves t2g orbitals degenerate • π donors decrease ∆oct • π acceptors increase ∆oct t1u* t2g* 4p a1g* empty π* 4s Ligands eg* eg* eg* eg* t2g* 3d t2g t2g π (filled) t2g Metal σ σ only t2g eg a1g Ligands t1u Octahedral ML6 Ligands π acceptor π donor 10Dq increases 10Dq decreases Jahn-Teller Effect • The d electrons are structurally and energetically non-innocent. • Complexes with a ground state orbital degeneracy unstable with respect to a vibration which removes the degeneracy - Jahn-Teller theorem dx2-y2 ∆EJT eg ∆EJT dz2 L L L -δ Cu L L L t2g +2δ Molecular Mechanics • Etot = ΣEstr + ΣEbend + ΣEtor + ΣEvdw + ΣEC 9 Fast (big systems, dynamics) 9 Accurate (experimental information built in to Force Field parameters) 8 Parameterised 9 Works well for organics and TM complexes with “regular” coordination environments 8 Problems with “plastic” systems 8 Problems with electronic effects Extending MM to the d-block • Problem: conventional MM requires independent FF parameters for high spin d8 (octahedral) Ni-N 2.1Å versus low spin d8 (planar) Ni-N 1.9Å • Answer: add LFSE directly to MM Ligand Field Molecular Mechanics (LFMM) • LFMM captures d electronic effects directly • Etot = ΣEstr + ΣEbend + ΣEtor + ΣEvdw + ΣEC + LFSE d-orbital energies • Crystal Field Theory is global symmetry approach – all ligands simultaneously • MM is bond centred • Need to express d orbital energies as function of individual bonds • Angular Overlap Model describes each bond´s contribution to the total ligand field potential Getting LF Parameters • Each M-L bond is described by up to three parameters — eσ, eπx, eπy. L L L M M Z M X Y dz2 dxz eσ d d dyz eπx d eπy Angular variations • d orbital energies for linear ligator M-L Z dz2 y x eσ(L) L Y z M Effect of moving ligand Fσ(dz2) = 1/4(1+3cos2θ) E(dz2) = eσ F(dz2) = 1/16 eσ (1 + 3cos2θ)2 _ d X θ = 0° Z L eπ(L) dxy,dx2-y2 Z Z θ = 25° L θ = 54.7° θ = 90° L M M M M . 1.00 0.75 Fraction of e(sigma) • • • dxz,dyz 0.50 0.25 0.00 0 30 60 90 θ 120 150 180 L Other motions Z θ = 0° θ = 25° L L χσ θ = 45° θ = 90° L M M X M M dxz 1.00 Fraction of e(sigma) 0.75 0.50 0.25 0.00 0 30 60 90 θ 120 150 180 L Octahedral symmetry Angular Coordinates Z y ψ θ z M N Y φ X Z x Ligand θ φ 1 90 0 2 90 90 3 0 0 4 90 180 5 90 270 6 180 0 L3 L4 Y L2 M L5 L1 L6 X d orbital energies • The energy of each d function will consist of the sum of all possible symmetry contributions (σ, πx, πy) from each ligand. For N ligands, this will in general correspond to a sum of 3N terms. • E(dz2) = ¼eσ(L1) + ¼ eσ(L2) + eσ (L3) + ¼ eσ (L4) + ¼ eσ (L5) + eσ (L6) = 3eσ (L) • E(dx2-y2) = ¾ eσ(L1) + ¾ eσ(L2) + 0eσ (L3) + ¾ eσ (L4) + ¾ eσ (L5) + 0eσ (L6) d ,d = 3eσ (L) ∆ 3e • AOM automatically recovers correct symmetry d ,d ,d oct 4eπ 'mean' d z2 x2-y2 xz yz σ xy Strategy and Examples • Only develop parameters for metal-ligand bonds • Use existing force fields for ´spinach´ • [CoF6]3– High spin d6 • [Co(CN)6]3– Low spin d6 • [CuCl4]2• Ammonia and amine complexes DFT Protocol for Bond Lengths • Optimised Bond lengths for [CoL6]3complexes Co-F Co-CN DFT(hs) 1.97 2.12* Exp 1.94 DFT(ls) Exp 1.88* - 1.88 1.89 • We can use the bond lengths for high-spin [Co(CN)6]3- and low-spin [CoF6]3- to design better LFMM parameters. Adding Chemical Unrealism: LFSE-free • MM uses separate energy terms so it is feasible to pose questions like “What is the M-L distance in the absence of LFSE?” • LFSE = 0 if all d orbitals equally occpied • For d6 Co(III), this corresponds to t2g3.6eg2.4 • DFT gives approximate LFSE-free bond length Adding Chemical Realism: π Bonding Both F- and CN- can form π bonds. Averaged configuration DFT calculations on hypothetical CoL4 species yields ‘d’ orbital energies which can be fitted to standard AOM expressions to determine eπ to eσ ratio. Co-F: ~0.3 Co-CN: ~0.1 (CN π donor!) Parameter Fitting • In general, we want to be able to handle large M-L bond length changes: use Morse function. • Angular geometry determined by 1,3-ligand-ligand (POS, VSEPR) interaction (plus LFSE contribution). • The required bond length, r, is a balance of Morse function (D0, α and r0) with the LFSE and POS. NB: r0 > r • CAN´T USE METAL PARAMETERS FROM OTHER FFs 160 Morse 120 CLFSE Energy 80 Total 40 0 1.50 -40 1.70 1.90 2.10 -80 -120 Bond Length 2.30 2.50 MOE • Scientific Vector Language • LFSE and derivatives: code written in C • Connect LFSE code to MOE via API and SVL communication routine function __LFMM_potential [x, args] // (nf) *********************************************************************** local function LFMM_potential; local [f,g] = LFMM_potential [lfmm_vector, x, args, 1]; // type 1 = optimisation, type2 = single point //*************************************************************************** return [f,g]; endfunction DommiMOE •D-orbitals in molecular mechnics in inoragnics in MOE