* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Qualitative assessment: Comparing Imaging features of primary
Dual consciousness wikipedia , lookup
Donald O. Hebb wikipedia , lookup
Cortical stimulation mapping wikipedia , lookup
Radiosurgery wikipedia , lookup
Brain damage wikipedia , lookup
Transcranial Doppler wikipedia , lookup
Neuroendocrine tumor wikipedia , lookup
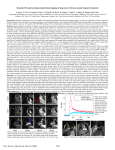
Multimodality Imaging, Qualitative Assessment Qualitative assessment: Comparing Imaging features of primary brain tumors utilizing MRI perfusion and Methionine-Positron Emission Tomography with respect to histopathological diagnosis. Nathan T. Mortensen, D.O., Arash Kardan, M.D., Victor Heh, Ph.D. Kettering Health Network/Grandview Medical Center Introduction Magnetic Resonance Imaging (MRI) perfusion has been increasingly utilized as a tool for brain tumor characterization. MRI perfusion can be a valuable imaging modality in that it adds a physiologic element to traditional Magnetic Resonance (MR), which is an exquisite diagnostic tool for assessment of anatomical abnormalities. Methionine-Positron Emission Tomography (Met-PET) is a very valuable imaging modality for the evaluation of brain tumors given its preferential uptake in neoplastic tissue, and relative lack of uptake in normal background brain parenchyma. Studies have shown that these imaging modalities have value. Many studies investigating these imaging methods involve quantitative calculations. Objective Can we make qualitative assessments of primary brain tumors utilizing of Methionine PET and MR perfusion? To further elucidate imaging features of primary brain tumors utilizing these imaging modalities. Methods This is a retrospective study. Patient charts were reviewed from from July 1, 2012 – July 30, 2015. Approval was obtained from the IRB, Kettering Health Network. Following IRB approval, a list of 81 patients was provided from the Oncology Outcomes department, Kettering Health Network, Dayton, Ohio. The list contained patients with histologically proven primary brain tumors. Inclusion criteria Patient age ranged from 18-89 years. Patients must have had a primary brain tumor, and the patient must have had imaging of the tumor with both a Methionine-PET and MRI perfusion prior to biopsy or surgical intervention of the tumor. All images reviewed for the study were the first set of MR with perfusion, and Met-PET images pertinent to the patient’s diagnosis in their imaging timeline. Exclusion criteria: Metastatic disease was not included in the study. The patient must have had both an MRI with perfusion, and a Met-PET. If the patient had an MR perfusion but no Met-PET, or vice versa, the study was not included. Post surgical images involving the lesion were not included. Discussion Figure 1: Glioblastoma Multiforme (WHO Grade IV) FLAIR, T1C+, 11-C-Methionine PET co-registered to MR, and MR perfusion K2 permeability and Cerebral Blood Volume (CBV) images. There is features of increased FLAIR signal, and peripherally enhancing lesion post contrast administration, with suggestion of central necrosis. 11-C-Met-PET shows hypermetabolic uptake relative to contralateral presumed normal brain. MR perfusion shows concomitant increased permeability on K2 in the region of hypermatabolic activity, particularly at the area of peripheral enhancement which suggests blood brain barrier disruption. There is no definite increased CBV relative to contralateral cortex, but it does appear equal to background regional to the lesion. Methods (continued) Image interpretation: All images were reviewed and interpreted by Nathan Mortensen, D.O. (PGY-4, third year radiology resident). The reader was blinded to the histological diagnosis during all image interpretation. Met-PET images were reviewed, along with anatomical MRI images, with emphasis on FLAIR, T1 post-contrast (T1C+), and then MRI perfusion images with emphasis on K2 permeability and cerebral blood volume (CBV). All Met-PET studies were co-registered to the most recent MR. A qualitative assessment of the Met-PET study was made with any focal hypermetabolic areas being given a score of +1 on visual inspection. Any Isometabolic areas were given a score of 0, and any hypometabolic areas were given a score of -1 on visual inspection. The reference for the MetPET studies would be presumed normal contralateral brain, particularly with respect to the specific lobe or lobes of the brain involved by tumor and how it related to the contralateral lobe. For the MR perfusion, K2 permeability and cerebral blood volume (CBV) maps were assessed. In a similar fashion to the Met-PET images, qualitative analysis was made by visual inspection comparing presumed normal contralateral brain on the permeability and CBV maps. Again, numerical values were given based on background perfusion. If the tumor showed high permeability (K2) or cerebral blood volume (CBV), it was given a score of +1 respectively. If it appeared homogeneous to background permeability it was scored as 0, and if it showed decreased permeability it was scored as a -1. Tumor sizes were measured in the maximum cross sectional diameter. Once imaging features were documented, they were compared to their respective histology. Figure 2. Glioblastoma Multiforme (WHO Grade IV) FLAIR, T1 C+, Co-registered MR with Methionine PET, MR perfusion K2 and CBV maps. Flair hyperintense, heterogenously enhancing, Met-PET hypermetabolic lesion demonstrates increased K2 permeability at the lesion and accompanying increased CBV. Figure 3: Anaplastic Astrocytoma (WHO Grade III) FLAIR hyperintense signal at the periphery, T1C+ hypointense, non-enhancing left temporal lobe lesion. Met-PET co-registered to MR demonstrates hypermetabolic activity in the anterior periphery of the lesion relative to contralateral presumed normal brain. MR permeability appears low to background as does CBV. Figure 3: Oligoastrocytoma (WHO Grade II) FLAIR hyperintense, T1 hypointense, T1C+ hypointense non-enhancing right frontal lobe lesion. Met-PET co-registered to MR shows hypermetabolic activity in the lesion relative to presumed normal contralateral parenchyma. MR perfusion shows background on K2 permeability map, and evidence of low CBV. Limitations: Patient sample size is too small to conduct valid statistics. This is a retrospective study. The images were interpreted qualitatively, which in and of itself can pose limitations, such as inter-observer variability. Furthermore, images were interpreted by a radiology resident. The strength of the study may have been improved with image interpretation carried out by those who have fellowship training in neuroradiology and nuclear medicine. Imaging parameters may have fluctuated over the time interval of the study as well, given the lack of a prospective model. In addition, the study is limited by its very small sample size. However, it is difficult to find substantial population sizes for such specialized imaging. References: Results All 9 brain tumors showed hypermetabolic activity on MetPET at the tumor site relative to presumed normal contralateral brain. All grade IV astrocytomas, 4/4 (100%) showed increased K2 permeability relative to presumed normal contralateral brain, and 3/4 (75%) of grade IV astrocytomas showed increased cerebral blood volume with respect to the contralateral parenchyma. There was 1 anaplastic astrocytoma, WHO grade III neoplasm, which demonstrated low permeability and volume on K2 and CBV respectively, but it was hypermetabolic on Met-PET. The four remaining tumors are WHO grade II, and 3/4 (75%) demonstrated background K2 permeability, while one oligoastrocytoma appeared to show lower permeability with respect to contralateral and background parenchyma. 2/2 oligoastrocytoma’s (100%) demonstrated lower CBV than contralateral or background brain. The Met-PET proved very valuable in the detection of tumor. All tumors that fit the inclusion criteria showed hypermetabolic activity at the lesion site relative to presumed normal contralateral brain. However, out of the 9 tumors imaged, there were 4 different histologies. Thus, Met-PET did not differentiate histology or grade of tumor. However, the additional information gained from the perfusion studies helped differentiate high grade from low grade tumors. For example, all four of the grade 4 astrocytoma’s demonstrated increased permeability on K2, and 3 out of these 4 tumors showed increased cerebral blood volume, suggesting both leaky capillaries and increased vascularity respectively. The suggestion of leaky capillaries on the K2 map coincides with the fact that all four grade IV tumors demonstrated increased enhancement on post-contrast imaging, which implies breakdown of the blood brain barrier. There was one grade IV tumor that showed background CBV, and demonstrated peripheral enhancement. There were no low grade tumors (WHO grade II) that showed increased permeability on K2. This further supports existing research that shows an increase in permeability is often associated with a high grade tumor, and is likely related to abnormal leakiness of capillaries. This study shows that with the use of multimodality imaging combining Methionine PET and MR perfusion, one can detect high metabolic activity in brain tumors and subsequently make more qualitative assessments about brain tumor grade with the addition of MR perfusion. This study points to high grade tumors demonstrating increased k2 permeability and CBV, while low grade show no increase in K2 or CBV. These are features which have been demonstrated in other papers, and this data further supports an expanding field in advanced neuroimaging, where information gained from PET and MRI perfusion can help better diagnose and stage patients. More studies which investigate these imaging modalities in a prospective manner may be helpful to further establish the strength of the data, and better define with what degree of certainty we can differentiate high grade from low grade neoplasm with imaging alone, which may help with staging of patients as well as neurosurgical planning. The results are compelling, and may be similar in a larger sample size. Figure 4: Oligodendroglioma (WHO Grade II) FLAIR hyperintense, T1C+ hypointense non-enhancing right side lesion overlapping lobes. Met-PET is hypermetabolic relative to contralateral brain. MR perfusion shows background permeability throughout, without evidence of focal increased permeability. The CBV map was interpreted as background in the study, but may be on the border of increased. However, there are similar patchy increased areas in the cortex seen on the CBV map, particularly near the left periventricular white matter. It was felt that this area did not stand out enough to be called increased. Patient Size (cm) FLAIR Size (cm) T1 precontrast Size (cm) T1 C+ Postcontrast MR enhancement K2 Permeability Cerebral Blood Volume CBV 11-Met PET Pathology WHO Grade 1 3.9x3.6 3.2x3.6 3.1x2.5 Peripheral ( +1 ) (0) ( +1) Glioblastoma IV 2 4.9x7.3 4.8x3.5 5.2x3.8 Peripheral ( +1 ) ( +1) ( +1 ) Glioblastoma IV 3 4.1x3.7 4.2x3.0 4.1x3.2 Heterogeneous ( +1 ) ( +1 ) ( +1) Glioblastoma IV 4 7.5x5.3 2.1x1.4 1.7x2.3 Heterogeneous ( +1 ) ( +1 ) ( +1 ) Glioblastoma IV 5 4.3x7.2 3.5x5.0 3.5x5.0 Minimal peripheral ( -1 ) ( -1 ) ( +1 ) Anaplastic Astrocytoma III 6 2.4x5.7 4.3x1.8 4.3x1.8 None apparent (0) ( -1 ) ( +1 ) Oligoastrocytoma II 7 8.3x5.6 8.3x4.7 8.3x4.7 None apparent ( -1 ) (-1) ( +1 ) Oligoastrocytoma II 8 6.1x3.5 5.3x3.4 5.3x3.4 None apparent (0) (0) ( +1 ) Oligodendroglioma II 9 2.9x3.0 2.8x3.9 2.8x3.9 Minimal (0) (0) ( +1 ) Oligodendroglioma II Met-PET: Hypermetabolic = +1, isomeatolic = 0, Hypometabolic = -1 MR K2 permeability: increased = +1, background = 0, decreased = -1 MR Cerebral Blood Volume (CBV): increased = +1, background = 0, decreased = -1 1. Mills SJ, Patankar TA, Haroon HA, et al. Do cerebral blood volume and contrast transfer coefficient predict prognosis in human glioma? AJNR Am J Neuroradiol 2006;27:853–58 2. Sugahara T, Korogi, Y, Kochi, M, et al. Correlation of MR imaging determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. AJR Am J Roentgenol 1998; 171: 1479-1486 3. Zhao, C., Zhang, Y., Wang, J. A meta-analysis on the diagnostic performance of 18F-FDG and 11C-Methionine PET for differentiating brain tumors. American Journal of Neuroradiology 2014; 35: 1058-1065 4. Kleihues P, Burger PC, Scheithauer BW. The new WHO classification of brain tumours. Brain Pathol 1993; 3:255-68 5. Wang X, Hu X, Xie P, Li W, Li X, Ma L. Comparison of magnetic resonance spectroscopy and positron emission tomography in detection of tumor recurrence in posttreatment of glioma: A diagnostic meta-analysis. Asia-Pacific J Clinical Oncol 2015 Jun; 11: 97-105 6. Fatterpekar GM, Galheigo D, Narayana A, Johnson G, Knopp E. Treatmentrelated change versus tumor recurrence in high-grade gliomas: a diagnostic conundrum—use of dynamic susceptibility contrast-enhanced (DSC) perfusion MRI. AJR. 2012;198:19–26. 7. Provenzale JM, Wang GR, Brenner T et al. Comparison of permeability in highgrade and low-grade brain tumors using dynamic susceptibility contrast MR imaging. AJR Am J Roentgenol 2002;178:711–716. 8. Covarrubias DJ, Rosen BR, Lev MH: Dynamic magnetic resonance perfusion imaging of brain tumors. Oncologist. 2004, 9: 528-537. 10.1634/theoncologist.95-528. 9. Smirniotopoulos, J.G., Murphy, F.M., Rushing, E.J. et al, Patterns of contrast enhancement in the brain and meninges. Radiographics. 2007;27:525–551. 10. Law M, Yang S, Babb JS et al. Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol. 2004;25(5):746–755.