* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 1 - University Corporation for Atmospheric Research
Survey
Document related concepts
Transcript
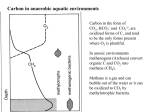
Global Change Instruction Program Biogeochemical Processes Innumerable biological, geological, and chemical processes cycle elements throughout the ecosphere. The few discussed in this section should give the reader an idea of their variety and complexity. As an example, consider a group of organisms called the prokaryotes: the bacteria and bluegreen algae. The processes that these organisms are involved with (summarized in Table 1) include: • methane production and oxidation • sulfur reduction and oxidation • nitrogen fixation, nitrification, and denitrification. This list is given only as an example; some of these processes will not be discussed in the text. These prokaryotic processes may take place in a variety of ways, such as (1) autotrophy, in which the organisms convert inorganic carbon in the environment to organic matter; (2) heterotrophy, in which the products from the breakdown of organic compounds are used to make new organic materials; and (3) mixotrophy, in which both inorganic and organic compounds are used to make organic matter. • the capture of carbon dioxide from the atmosphere and its conversion to organic matter (fixation of CO2) • the release of CO2 back to the atmosphere (through respiration and decay) • fermentation of sugar Table 1. Biogeochemical reactions involving prokaryotes Element Process Summary of partial chemical reactions Examples of organisms involved in process Carbon CO2 fixation CO2 + H2 ⇒ (CH2O)n + A2 (A = O, S) Photoautotrophs: cyanobacteria, purple and green sulfur bacteria Chemoautotrophs: sulfur and iron oxidizing bacteria Methanogenesis COO- + H2 ⇒ CH4 Methanogenic bacteria Methanotrophy CH4 + O2 ⇒ CO2 Methanotrophic bacteria Fermentation (CH2O)n + O2 ⇒ CO2 Anaerobic heterotrophic bacteria Respiration (CH2O)n + O2 ⇒ CO2 Aerobic heterotrophic bacteria Sulfur reduction SO4 + H2 ⇒ H2S Sulfur-reducing bacteria Sulfur oxidation H2S ⇒ S0 Purple and green sulfur phototrophs S0 + O2 ⇒ SO4 Sulfur oxidizing bacteria N2 fixation N2 + H2 ⇒ NH4 Phototrophic bacteria, nitrogen-fixing heterotrophic bacteria Nitrification NH4 + O2 ⇒ NO2, NO3 Nitrifying bacteria Sulfur Nitrogen Denitrifying bacteria Denitrification NO2, NO3 ⇒ N2O, N2 __________________________________________________________________ After Stolz et al., 1989 3 Understanding Global Change: Earth Science and Human Impacts Principles of chemical reactions Atoms and elements Every object in the universe is composed of matter. Because matter can be converted to energy, it is essentially a form of energy. Matter is composed of atoms, which are the smallest particles of an element that can exist either alone or in combination. An atom is also the smallest particle that can enter into a chemical reaction. Most atoms never change; they only combine with other atoms to make different substances. Radioactive atoms, however, do change and eventually decay into stable, nonradioactive atoms. Elements consist of atoms of the same kind and, when pure, cannot be decomposed by a chemical change. There are 106 known elements; 103 are listed in the periodic table (Figure 2). The elements most used commercially by people, in order of use, are carbon (C), in the form of coal, oil, and gas; sodium (Na), in table salt and other products; iron (Fe), used in the steel industry; and nitrogen (N), sulfur (S), potassium (P), and calcium (Ca), all used in fertilizers or as soil conditioners for our food supply. Compounds When two or more atoms are bonded together in a definite proportion, a compound is formed. Examples of compounds discussed in this text are water (H2O), carbon dioxide (CO2), salt (NaCl), and sugar (e.g., glucose, C6H12O6 ). (All of the compounds named in the text are listed in Table 2.) The numbers in these chemical formulas are the number of atoms of each substance in the compound. If only one atom of a substance is in the compound, no number is given. The universe is composed of millions of these compounds, all created from the elements given in the periodic table. The smallest particle of a compound that can exist and exhibit the properties of that compound is called a molecule. A compound is a pure substance that can be decomposed by a chemical change. The atoms in the chemical compound may rearrange themselves, or they may separate from the compound to form different compounds. These changes and interactions among compounds are called chemical reactions. Chemical equations A chemical equation expresses a chemical reaction involving compounds or elements. The chemicals that react together, called reactants, generally are shown on the left-hand side of the equation and the products on the right-hand side. Consider the decay of plant material (represented by the chemical compound CH2O, a carbohydrate), which requires the oxygen gas (the chemical compound O2) in the earth’s atmosphere. The simplest chemical equation representing this process is CH2O + O2 ⇒ CO2 + H2O (1) The arrow pointing right indicates that this process is irreversible; the plant material will be completely oxidized to CO2 and H2O in the presence of atmospheric oxygen. Other processes are highly reversible, and these are usually represented by a double arrow. For example, the equilibrium between calcium carbonate and its dissolved calcium and carbonate ions (atoms or molecules that have lost or gained electrons, with the number lost or gained shown as a positive or negative superscript) is represented as CaCO3 ⇔ Ca2+ + CO32- (2) In chemical processes, matter cannot be created or destroyed. Thus, when a chemical equation is written, the total number of atoms of any particular element on the left-hand side of a chemical equation must be made to equal the total number of atoms of that element on the right-hand side of the equation. This is the process of balancing a chemical equation. Balancing the equation expresses the fact that molecules usually react in such a way as to bear simple, integral, numerical relationships to one another. 4 Global Biogeochemical Cycles and the Physical Climate System If these relationships are known, it is possible to calculate the masses of reactants and products by using known atomic and molecular weights. In chemical terms, the amount of a substance is expressed in moles. One mole of a substance is the amount that contains as many elementary entities as there are atoms in 12 grams of carbon. This number is termed Avogadro’s constant, and its value is equal to 6.022 x 1023. In the chemical equation given above for the equilibrium of CaCO3 and its dissolved chemical species, one mole of CaCO3 will dissolve in water to make one mole of Ca2+ and one mole of CO32-. In terms of mass, 100 grams of CaCO3 will react to give 40 grams of Ca2+ and 60 grams of CO32-. If only 10 grams of CaCO3 were to dissolve, then the same proportions of Ca2+ and CO32- would be present at the equilibrium: 4 and 6 grams, respectively. Figure 2. Periodic table of the elements. Each box includes an element’s atomic number, chemical symbol, and atomic weight. 5 Understanding Global Change: Earth Science and Human Impacts Table 2. Chemical formulas and names used in this module Al2Si2O5(OH)4 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .kaolinite Ca2+ . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .calcium ion CaCO3 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .calcium carbonate CaSiO3 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .calcium silicate Ca5(PO4)3(OH,F) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .carbonate fluoroapatite CH2O . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .carbohydrate (CH2O)106(NH3)16H3PO4 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .organic matter in marine phytoplankton CH4 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .methane CO2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .carbon dioxide CO32- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .carbonate ion CS2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .carbon disulfide C6H12O6 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .sugar (glucose) DIC . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .dissolved inorganic carbon DMS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .dimethyl sulfide, (CH3)2S HCO3- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .bicarbonate ion HNO3 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .nitric acid H2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .molecular hydrogen H2O . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .water H2S . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .hydrogen sulfide H2SO4 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .sulfuric acid H3PO4 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .phosphoric acid H4SiO40 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .monomeric silicic acid KAlSi3O8 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .orthoclase feldspar MSA . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .methane-sulfonic acid NaAlSi3O8 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .albite NaCl . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .sodium chloride, common table salt NH3 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .ammonia NH4+ . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .ammonium ion NH4NO3 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .ammonium nitrate (NH4)2SO4 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .ammonium sulfate NMHC . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .nonmethane hydrocarbon NO . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .nitric oxide NO3- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .nitrate ion NOx . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .oxides of nitrogen N2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .diatomic nitrogen N2O . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .nitrous oxide OCS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .carbonyl sulfide OH* . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .hydroxyl radical OH- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .hydroxyl ion O2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .diatomic oxygen (pure oxygen molecules) O3 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .ozone PAN . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .peroxylacetyl nitrate PH3 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .phosphine or swamp gas PO43+ . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .phosphate ion SO2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .sulfur dioxide SO42- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .sulfate ion SOx . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .oxides of sulfur SiO2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .silica 6 Global Biogeochemical Cycles and the Physical Climate System Incidentally, the carbon dioxide and donor molecule used for photosynthesis are not the only requirements for plant growth. Plants also need nitrogen, phosphorus, sulfur, potassium, and a dozen or so trace elements, like zinc and iron. As we shall see below, human activities are changing the atmospheric concentrations of these nutrients as well as that of carbon, with various possible effects on plants. The photosynthetic reactions that produce organic matter on land differ from those in the ocean because the proportions of carbon, nitrogen, sulfur, and phosphorus in land vegetation differ from those in marine plankton. The ratio of C:N:S:P in marine plankton is 106:16:1.7:1. Known as the Redfield ratio, this proportion is fairly constant for the surface-dwelling, microscopic plants (phytoplankton) of the world’s oceans. The C:N:S:P ratio for land plants is more variable but averages 882:9:0.6:1. The amount of carbon is so much greater in land vegetation because it is stored as cellulose in the structural tissues of trees and grasses. From this summary, it can be seen that photosynthesis (among other things) links the biogeochemical processes and cycles of the individual organic elements of carbon, nitrogen, phosphorus, and sulfur. These elements, plus hydrogen and oxygen, are the major constituents of organic matter. Those six elements and about a dozen or so minor elements are necessary for the maintenance of organic structures and the physiological functions of living organisms. Photosynthesis We begin with perhaps the most important biogeochemical process of all, photosynthesis. It is a photoautotrophic process, that is, an autotrophic reaction in the presence of light. Nutrients such as phosphate (PO43-) and nitrate (NO3-) are also necessary for this reaction to occur. In the early stages of our planet’s formation, the atmosphere was very different from that of today. There was no free molecular oxygen (O2), which most of today’s life forms require. In fact, oxygen was a very powerful poison for the simple organisms that lived in this early, oxygendeficient (anaerobic) world. Both the organisms and the earth had to evolve to a stage where the organisms produced oxygen and emitted it to their environment before more advanced life forms could evolve. Photosynthesis, the process of constructing complex organic molecules from simple inorganic ones in the presence of light, was a critical step in the evolution of life and allowed the mass of living organisms to grow to the level of today. In our world, the mass of living organisms on earth is equivalent to about 600 billion tons of carbon. More than 99% of this carbon is in land plants; the remainder is stored in marine plants and in animals. Photosynthesis is basically a chemical reaction or process in which carbon-, hydrogen-, and oxygen-bearing chemical compounds (carbohydrates) are synthesized from atmospheric CO2 and H2O or another chemical compound that can act as a hydrogen donor. The generalized reaction is Respiration and Decay of Organic Matter energy + nCO2 + 2nH2A ⇒ (CH2O)n + (3) nH2O + 2nA In the life cycle, photosynthesis in plants is balanced by the complementary processes of respiration and decay in plants and animals. In plants, respiration is the breakdown of the complex organic molecules that were formed during photosynthesis. The chemical reactions for respiration and decay are the reverse of those shown above for the production of organic material. The generalized reaction is C6H12O6 + 6H2O + 6O2 ⇒ 6CO2 + 12H2O + energy (5) where H2A is a hydrogen donor molecule, (CH2O) is a carbohydrate, and n stands for any number. In higher plants, the donor molecule is water, and n = 6. Thus for these plants the specific reaction is energy + 6CO2 + 12H2O ⇒ C6H12O6 + 6H2O +6O2 (4) For photosynthetic sulfur bacteria the donor molecule is hydrogen sulfide (H2S), and for nonsulfur purple bacteria it is organic compounds. DRAFT 7 Understanding Global Change: Earth Science and Human Impacts Compare this with reaction 4. The amount of energy released is about 686 kilocalories (kcal) for each mole of C6H12O6 (glucose, the most common form of sugar in living things) that is broken down. In animals, the respiratory oxidation of foods —that is, the loss of electrons from the carbon in carbohydrates, occurring during digestion— provides energy for a variety of uses, including maintenance of body temperature, muscular movement, and synthesis of complex organic compounds. During the oxidation of organic matter, CO2, nitrogen- and phosphorus-bearing nutrients, and bioessential trace elements (e.g., iron) are returned to the environment to be used again in the production of more organic matter. When O2 is available, it is the oxidizing agent (oxidant); however, in oxygen-depleted (anoxic) waters, sediments, and soils, other oxidants are used. These include nitrate, sulfate, and iron and manganese oxides. The chemical equations for respiration and decay, either in an oxygenated or in an anoxic environment, are more complex than the generalized reaction for photosynthesis given above. For example, the chemical composition of average marine phytoplankton—a relatively simple form of life—is (CH2O)106(NH3)16H3PO4: 106 molecules of carbohydrate, 16 of ammonia, and 1 of phosphoric acid. When dead phytoplankton react with O2 in an oxygenated environment, the products are carbon dioxide, nitric acid, phosphoric acid, and water: Weathering of Rocks Another very important set of biogeochemical processes is that involved with the breakdown of rocks exposed to rain, wind, and ice. Weathering prepares rock for erosion and transportation. Its products are dissolved chemical species and solids derived from changes in the primary minerals of the rock being weathered. The solid products are predominantly clay minerals; there are also dissolved products, predominantly calcium, carbon, and silicon. Ultimately, the products of weathering are either carried by water, blown as dust, or carried by glaciers to the ocean. Of the approximately 20 billion tons of solids and dissolved materials reaching the ocean annually from the land, more than 80% is delivered by rivers. However, high-temperature chemical reactions in the presence of seawater along the great submarine midocean ridges are significant sources of dissolved calcium, silica, and iron for the oceans. An example of a chemical weathering reaction is the weathering of the mineral albite (the inorganic chemical compound NaAlSi3O8), found in igneous rocks like basalt, to the clay mineral kaolinite [Al2Si2O5(OH)4]. The reaction takes place principally in the presence of soil water and groundwater that contain significant amounts of dissolved CO2. Although the ultimate source of the CO2 is the atmosphere, much of it does not come directly from the air but is produced in soils by the respiration of plants and the decay of dead plants and animals. Because of these processes, the concentration of CO2 in soils may be one or more orders of magnitude greater than that of the atmosphere. The elevated CO2 levels give rise to acidic soil solutions, and these corrosive, low-pH soil solutions are responsible for the weathering of rock minerals like albite: (CH2O)106(NH3)16H3PO4 + 138O2 ⇒ 106CO2 + 16HNO3 + H3PO4 + 122H2O (6) For an example of respiration and decay in an anoxic environment, let us consider the reduction of sulfur in sulfate (SO42-) in the pore waters of anoxic sediments. Bacteria use the oxygen originally bound in the sulfate to oxidize organic matter. Again using phytoplankton as the organic matter, the equation for this chemical reaction is 2NaAlSi3O8 + 2CO2 + 11H2O ⇒ Al2Si2O5(OH)4 + 2Na+ + 2HCO3- + 4H4SiO40 (8) (CH2O)106(NH3)16H3PO4 + 53SO42- ⇒ 106CO2 + 16NH3 + H3PO4 + 53S2- + 106H2O (7) The products of this reaction, besides the kaolinite, are sodium ion, bicarbonate ion, and monomeric silicic acid. In regions where human activities such as coal burning release considerable amounts of sulfur and nitrogen oxide gases to the atmosphere, This time, in addition to carbon dioxide, phosphoric acid, and water as in reaction 6, the products include ammonia (NH3) and sulfide (S2-). 8 Global Biogeochemical Cycles and the Physical Climate System inorganic carbon brought to the oceans annually by rivers. The other half of the riverborne carbon is released to the ocean and atmosphere when skeletal carbonate minerals are formed. Dissolved silica is also removed from the oceans in the skeletons of marine organisms. Certain of these organisms—planktonic diatoms (algae), radiolarians (protozoans), dinoflaggelates (protozoans), and benthic sponges—use dissolved silica to form their shells of opaline silica. After these organisms die, most of the opal dissolves, because the oceans throughout their extent are undersaturated with respect to this chemical compound. Only about 40% of the total annual production of skeletal silica sinks below the parts of the ocean that daylight reaches (the euphotic zone). Most of this siliceous material dissolves en route to the seafloor; only 5% of that produced in the euphotic zone accumulates in marine sediments. This amount is about equivalent to the annual input of dissolved silica to the oceans by rivers. such as the midwestern and eastern United States and southern China, the pH of rainwater and consequently soil water may be lower (more acid) than natural values. This happens because the gases oxidize and react with water in the atmosphere and then rain out as sulfuric and nitric acids, respectively. This phenomenon is the environmental problem of acid deposition (often called acid rain), which in extreme forms can be responsible for increased fish mortalities in lakes and decreased agricultural production. Deposition in the Oceans When the solid and dissolved products of weathering reach the ocean, the solids settle out because of their weight and are deposited on the seafloor as gravel, sand, silt, and mud. How long the dissolved products remain in the ocean depends on how long it takes them to enter into a chemical or biochemical reaction. As an example of the periods involved, dissolved sodium in the ocean has a long residence time, about 55 million years. At the other end of the time scale, the residence time of dissolved silica is only 20,000 years. Sodium and magnesium In contrast to carbon and silica, which are removed from the ocean primarily by biological processes, riverborne dissolved sodium and magnesium are removed to a significant extent by inorganic chemical reactions. Both of these elements are involved in hydrothermal reactions between seawater circulating through midocean ridges and the basalt rock making up the ridges. In the hydrothermal reaction process, sodium and magnesium are removed from the seawater. Sodium is also removed from the ocean by the precipitation of halite (common table salt, sodium chloride) from seawater. This process is very important as a removal mechanism for sodium and chlorine, but only occurs when the right set of climatic and tectonic conditions are achieved. Only seawater in relatively isolated arms of the sea can be sufficiently evaporated to reach halite saturation. Thus, because such environments are scarce today, it is likely that sodium and chlorine brought to the oceans by rivers are currently accumulating in seawater. Some magnesium is also removed from seawater by chemical processes in the pore waters of Calcium and silica Many of the processes by which dissolved constituents are removed from the ocean involve marine organisms. In today’s oceans, dissolved calcium and bicarbonate are precipitated as carbonate minerals in the skeletons of several kinds of marine organisms: planktonic foraminifera (protozoans), pteropods (mollusks), and Coccolithophoridae (algae), and bottom-dwelling (benthic) corals, echinoids, mollusks, and coralline algae. Of the total production of skeletal carbonate in the oceans, equivalent to about 1 billion tons of carbon per year, 80% is redissolved in the ocean as skeletal debris sinks to the seafloor. This efficient recycling is due to the fact that although the surface ocean is oversaturated with respect to calcium carbonate, the deeper sea is undersaturated with respect to this mineral. The remaining 20% of the ocean’s carbonate production accumulates in shallow-water and deep-sea sediments. The amount of carbon in these sediments only accounts for about one-half of the dissolved 9 Understanding Global Change: Earth Science and Human Impacts sediments. These processes taking place during the burial of sediments are collectively referred to as diagenesis. The relative importance of diagenetic and hydrothermal reactions for the removal of magnesium from seawater is a topic of current scientific research and debate. We can conclude from the above discussion that the circulation of material through the ecosphere is complex and involves myriad chemical, biological, and geological processes. The system is truly biogeochemical in nature. On all time and space scales, if the composition of the ecosphere is regulated, the regulation is controlled by a complex of interwoven inorganic and organic processes. The maintenance of the equable environment, including climate, that is required for life to exist on earth is a product of this interacting and interwoven web of biogeochemical processes and cycles. 10