* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Aldosterone, ion channels, and sudden death: another - AJP
Survey
Document related concepts
Remote ischemic conditioning wikipedia , lookup
Coronary artery disease wikipedia , lookup
Heart failure wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Electrocardiography wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Cardiac surgery wikipedia , lookup
Ventricular fibrillation wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Transcript
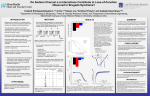
Am J Physiol Heart Circ Physiol 290: H2176 –H2177, 2006; doi:10.1152/ajpheart.00186.2006. Editorial Focus Aldosterone, ion channels, and sudden death: another piece of the circle? Geoffrey S. Pitt1 and Bertram Pitt2 1 Departments of Medicine and Pharmacology and Center for Molecular Cardiology, College of Physicians and Surgeons, Columbia University, New York, New York; and 2Department of Medicine, University of Michigan, Ann Arbor, Michigan, and William Beaumont Hospital, Department of Internal Medicine, Royal Oak, Michigan Address for reprint requests and other correspondence: G. S. Pitt, Columbia Univ., Dept. of Pharmacology, 630 W. 168th St., PH 7W 318, New York, NY 10032 (email: [email protected]). H2176 tricular myocardium and the bundle branches, as well as abnormal distribution of connexin 43, the gap junction responsible for propagation of electrical activity in ventricular myocytes (18, 21). Fibrosis may affect the conduction disturbances in BrS, too. A histopathological study of an explanted heart from a BrS patient showed extensive fibroelastosis and fatty infiltration within the right ventricle (6). Together, these suggest that a reduction in sodium channels might activate gene expression cascades that result in fibrosis. Indeed, the early growth response-1 transcription factor (Egr-1), a master regulator that influences the expression of many other genes, was found to be upregulated in the Scn5a⫹/⫺ mice. Nevertheless, there are several missing links in these signaling pathways, and their identification could open the possibility of pharmacological intervention. In this context, a report in this issue offers new insight and perhaps therapeutic promise. Measuring sodium channel currents in cultured mouse ventricular myocytes, Boixel et al. (5) in a study in this issue of the American Journal of Physiology-Heart and Circulatory Physiology found that incubation with aldosterone for 24 h increased current density in a dose-dependent manner and lengthened the action potential duration. This effect appeared dependent on the mineralocorticoid receptor (MR) because it was blocked with the MR antagonist spironolactone but not with the glucocorticoid receptor antagonist RU-38486. Confirming previous results from Bénitah and colleagues, this report also demonstrated that aldosterone, acting through the MR, increased Ca2⫹ channel current density (13) and fits well with a growing literature that shows how aldosterone influences the electrical properties of cardiac myocytes and increases action potential duration. These results are compelling for at least two reasons. First, aldosterone has been shown to activate a number of mediators that can lead to myocardial fibrosis (15). One only has to implicate a homeostatic process whereby decreased sodium channel current density triggers a feedback loop that increases aldosterone to appreciate how haploinsufficiency for SCN5A could lead to fibrosis. Indeed, Egr-1, the transcription factor upregulated in Scn5a⫹/⫺ mice, is activated specifically in the hearts of transgenic mice overexpressing the mineralocorticoid receptor but not in other aldosterone target tissues (10). Thus PCCD, the most common cause worldwide for pacemaker implantation, is ripe for testing whether aldosterone antagonism prevents or delays the development of conduction block. Second, we may now be seeing part of the molecular basis for the striking clinical finding that a significant portion of the mortality benefit of aldosterone antagonism in heart failure derives from a reduction in sudden death (14). Blockade of a mechanism that increases sodium channel density (this report) or increases calcium channel density and then decreases the transient outward current (Ito) (4) would ameliorate QT prolongation, a common finding and an independent risk factor for sudden death in heart failure (3, 19, 20, 22). 0363-6135/06 $8.00 Copyright © 2006 the American Physiological Society http://www.ajpheart.org Downloaded from http://ajpheart.physiology.org/ by 10.220.33.6 on May 5, 2017 of the genetic basis for many inherited and certain acquired cardiac arrhythmias during the last decade, the connection between identified mutations and consequent arrhythmias remains unclear in many cases. For some, the mechanisms for arrhythmogenesis are well understood. Lossof-function mutations in ether-à-go-go-related gene (HERG), the pore-forming subunit of inwardly rectifying K⫹ current (IKr), decrease the “repolarization reserve” and render patients susceptible to reentry-induced arrhythmias during the late phase of the action potential, thus forming the basis for Long QT Syndrome 2 (LQTS2) and certain acquired (drug-induced) Long QT Syndromes (17). Certain mutations in SCN5A, the gene for the cardiac sodium channel, affect how the channel inactivates; the mutant channels thereby generate a persistent inward depolarizing Na⫹ current during the action potential plateau phase, especially at slow heart rates, which forms the substrate for arrhythmia-triggering early afterdepolarizations in LQTS3 (8). For several arrhythmogenic disorders resulting from haploinsufficiency of the sodium channel, the age-related slowing of cardiac conduction that leads to atrioventricular block, which defines progressive cardiac conduction defect (PCCD) or a subset of the autosomal dominantly inherited Brugada syndrome (BrS), for example, the molecular basis has been more difficult to understand. There are at least two puzzling features about the connection between loss-of-function mutations in one SCN5A allele and the resultant arrhythmias. First, despite the presence of mutations that reduce the number of sodium channels from birth, patients with these disorders do not generally become symptomatic before the third or fourth decade of life. PCCD is clearly age related. Arrhythmogenic sudden death from BrS, the most common cause of death for men in Southeast Asia other than accidents (1), is rare in children; in a study of the natural history of BrS, the mean age at the time of the index cardiac event was 33 ⫾ 13 y (16). Second, modeling of the cardiac action potential suggests a large safety factor for the sodium current; reducing the sodium current even by one-half should not, in itself, lead to conduction slowing or block (9). What additional factor(s) contribute and why symptoms occur primarily in older patients are now becoming clear from a number of recent studies. Analysis of mice with a knockout of the SCN5A gene suggested that the development of fibrosis may be a major contributor to these arrhythmias (18). Homozygous Scn5a⫺/⫺ mice died before birth, but heterozygous (Scn5a⫹/⫺) mice (viable and fertile) showed slowing of cardiac conduction and various degrees of block as they aged, similar to patients with PCCD (12). Correlating with the appearance of conduction slowing was extensive heterogeneous fibrosis within the venDESPITE DISCOVERY Editorial Focus H2177 GRANTS 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. This paper was supported by National Heart, Lung, and Blood Institute Grant HL-71165 (to G. S. Pitt) and the American Heart Association Grant 0555875T. G. S. Pitt is a recipient of the Irma T. Hirschl Career Scientist Award and is the Aboodi Assistant Professor of Cardiology. 18. REFERENCES 1. Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, Gussak I, LeMarec H, Nademanee K, Perez Riera AR, Shimizu W, Schulze-Bahr E, Tan H, and Wilde A. Brugada Syndrome: report of the Second Consensus Conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation 111: 659 – 670, 2005. 2. Barr CS, Lang CC, Hanson J, Arnott M, Kennedy N, and Struthers AD. Effects of adding spironolactone to an angiotensin-converting enzyme inhibitor in chronic congestive heart failure secondary to coronary artery disease. Am J Cardiol 76: 1259 –1265, 1995. 3. Barr CS, Naas A, Freeman M, Lang CC, and Struthers AD. QT dispersion and sudden unexpected death in chronic heart failure. Lancet 343: 327–329, 1994. 4. Benitah JP, Perrier E, Gomez AM, and Vassort G. Effects of aldosterone on transient outward K⫹ current density in rat ventricular myocytes. J Physiol 537: 151–160, 2001. 5. Boixel C, Gavillet B, Rougier JS, and Abriel H. Aldosterone increases voltage-gated sodium current in ventricular myocytes. Am J Physiol Heart Circ Physiol 290: H2257–H2266, 2006. 6. Coronel R, Casini S, Koopmann TT, Wilms-Schopman FJG, Verkerk AO, de Groot JR, Bhuiyan Z, Bezzina CR, Veldkamp MW, Linnenbank AC, van der Wal AC, Tan HL, Brugada P, Wilde AAM, and de AJP-Heart Circ Physiol • VOL 19. 20. 21. 22. 23. Bakker JMT. Right ventricular fibrosis and conduction delay in a patient with clinical signs of Brugada Syndrome: a combined electrophysiological, genetic, histopathologic, and computational study. Circulation 112: 2769 –2777, 2005. Francis J, Weiss RM, Wei SG, Johnson AK, Beltz TG, Zimmerman K, and Felder RB. Central mineralocorticoid receptor blockade improves volume regulation and reduces sympathetic drive in heart failure. Am J Physiol Heart Circ Physiol 281: H2241–H2251, 2001. George AL Jr. Inherited disorders of voltage-gated sodium channels. J Clin Invest 115: 1990 –1999, 2005. Kleber AG and Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev 84: 431– 488, 2004. Le Menuet D, Isnard R, Bichara M, Viengchareun S, Muffat-Joly M, Walker F, Zennaro MC, and Lombes M. Alteration of cardiac and renal functions in transgenic mice overexpressing human mineralocorticoid receptor. J Biol Chem 276: 38911–38920, 2001. Olson TM, Michels VV, Ballew JD, Reyna SP, Karst ML, Herron KJ, Horton SC, Rodeheffer RJ, and Anderson JL. Sodium channel mutations and susceptibility to heart failure and atrial fibrillation. JAMA 293: 447– 454, 2005. Papadatos GA, Wallerstein PMR, Head CEG, Ratcliff R, Brady PA, Benndorf K, Saumarez RC, Trezise AEO, Huang CLH, Vandenberg JI, Colledge WH, and Grace AA. From the cover: slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene Scn5a. PNAS 99: 6210 – 6215, 2002. Perrier R, Richard S, Sainte-Marie Y, Rossier BC, Jaisser F, Hummler E, and Benitah JP. A direct relationship between plasma aldosterone and cardiac L-type Ca2⫹ current in mice. J Physiol 569: 153–162, 2005. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M, and Eplerenone PostAcute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 348: 1309 –1321, 2003. Pitt B, Stier CT Jr, and Rajagopalan S. Mineralocorticoid receptor blockade: new insights into the mechanism of action in patients with cardiovascular disease. J Renin Angiotensin Aldosterone Syst 4: 164 –168, 2003. Priori SG, Napolitano C, Gasparini M, Pappone C, Bella PD, Giordano U, Bloise R, Giustetto C, De Nardis R, Grillo M, Ronchetti E, Faggiano G, and Nastoli J. Natural history of Brugada syndrome: insights for risk stratification and management. Circulation 105: 1342– 1347, 2002. Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med 350: 1013–1022, 2004. Royer A, van Veen TAB, Le Bouter S, Marionneau C, Griol-Charhbili V, Leoni AL, Steenman M, van Rijen HVM, Demolombe S, Goddard CA, Richer C, Escoubet B, Jarry-Guichard T, Colledge WH, Gros D, de Bakker JMT, Grace AA, Escande D, and Charpentier F. Mouse model of SCN5A-linked hereditary Lenegre’s Disease: age-related conduction slowing and myocardial fibrosis. Circulation 111: 1738 –1746, 2005. Spargias KS, Lindsay SJ, Kawar GI, Greenwood DC, Cowan JC, Ball SG, and Hall AS. QT dispersion as a predictor of long-term mortality in patients with acute myocardial infarction and clinical evidence of heart failure. Eur Heart J 20: 1158 –1165, 1999. Tomaselli GF, Beuckelmann DJ, Calkins HG, Berger RD, Kessler PD, Lawrence JH, Kass D, Feldman AM, and Marban E. Sudden cardiac death in heart failure. The role of abnormal repolarization. Circulation 90: 2534 –2539, 1994. van Veen TAB, Stein M, Royer A, Le Quang K, Charpentier F, Colledge WH, Huang CLH, Wilders R, Grace AA, Escande D, de Bakker JMT, and van Rijen HVM. Impaired impulse propagation in Scn5a-knockout mice: combined contribution of excitability, connexin expression, and tissue architecture in relation to aging. Circulation 112: 1927–1935, 2005. Vrtovec B, Delgado R, Zewail A, Thomas CD, Richartz BM, and Radovancevic B. Prolonged QTc interval and high B-type natriuretic peptide levels together predict mortality in patients with advanced heart failure. Circulation 107: 1764 –1769, 2003. Yee KM, Pringle SD, and Struthers AD. Circadian variation in the effects of aldosterone blockade on heart rate variability and QT dispersion in congestive heart failure. J Am Coll Cardiol 37: 1800 –1807, 2001. 290 • JUNE 2006 • www.ajpheart.org Downloaded from http://ajpheart.physiology.org/ by 10.220.33.6 on May 5, 2017 A recent report (11) showing that loss-of-function mutations in SCN5A also underlie some forms of inherited dilated cardiomyopathy (DCM) makes it tempting to speculate even more broadly. Loss-of-function sodium channelopathies may represent different manifestations of a common starting point in which a reduction in sodium channels increases aldosterone. The distinct consequences inherent to specific syndromes could reflect the interactions of unique contributing factors that may derive, at least in part, from specific differences in effects of the mutations. “Loss-of-function” mutations (which implies that mutant channels, expressed in heterologous systems, fail to display the full complement of wild-type sodium channel functions) may produce channels that lack any inward current, channels that support a reduced inward current, or may instead affect channel trafficking, targeting, or interaction with other regulatory proteins. These defects may differentially alter the net sodium current or its kinetics, changing electrical activation and thereby the cardiac load within the complex milieu of the cardiac cycle so that over time the effects are mainly rightsided effects in some cases (BrS) or more global in others (DCM). The data in Boixel et al. (5) hint at this complexity: aldosterone increased sodium channel current density without changing channel protein or mRNA levels, but inhibitors of protein synthesis or trafficking blocked the increase, suggesting that aldosterone affected the expression and trafficking of a sodium channel regulatory protein. Under certain circumstances, aldosterone may also have other effects that could contribute to sudden death, including stimulation of central sympathetic drive (7), release of norepinephrine from peripheral sympathetic nerves (2), and a decrease in heart rate variability and baroreceptor function (23). Regardless, the new information reported by Boixel et al. (5) suggests that MR blockade may have an important role in reducing sudden death in a far broader spectrum of circumstances than hitherto thought possible.