* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Cumulative risk of developing VAP with the duration of mechanical
Survey
Document related concepts
Transcript
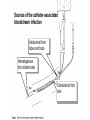
Need for a Global Approach Global burden: HAIs lead to excess morbidity, mortality, and healthcare costs worldwide Proliferation of invasive healthcare internationally without commensurate infection prevention infrastructure Antimicrobial resistance: everyone’s problem Global Burden Healthcare-associated infection (HAI) in the United States (2002) 1/20 patients 1.7 million HAIs 99,000 deaths Developing countries Limited data from low income countries Estimated prevalence: at least three times greater than United States Klevens et al Public Health Reports 2007. Allegranzi et al Lancet 2011. Why might there be more HAIs in middle- and low-income countries? Less infection prevention and control infrastructure Training lacking in general infection control Improper use of equipment (e.g., reuse of single-use equipment) Insufficient reprocessing Less surveillance, awareness, and targeted prevention efforts Proliferation of invasive medical care across the globe Large dialysis organizations expanding across boarders Increase in medical tourism Antimicrobial Resistance Studies suggest that approximately ½ of antimicrobial use in US healthcare settings is inappropriate Rising resistance leads to decreasing treatment options and increasing cost Inappropriate prescribing contributor to C. difficile epidemic Klebsiella Pneumoniae Carbapenemase KPC confers resistance to all b-lactams including extended-spectrum cephalosporins and carbapenems Is the predominant mechanisms of carbapenem resistance in Enterobacteriaceae (CRE) in the US. Mortality-associated with Resistance OR 3.71 (1.97-7.01) OR 4.5 (2.16-9.35) Patel et al. Infect Control Hosp Epidemiol 2008;29:1099-1106 Multidrug Resistant Organism (MRO) • Multidrug resistant organisms of concern at PSMH are Methicillin Resistant Staphylococcus Aureus (MRSA), Vancomycin Resistant Entercoccus (VRE) and Clostridium Difficile (C. Dif) • MRO’s are bacteria that have become resistant to many of the antibiotics used to treat infections caused by them. 7 Clostridium Difficile Patients admitted with diarrhea or develop diarrhea after admission are placed in Contact Isolation until C. dif is ruled out and Infection Control Department discontinues isolation. Positive C.dif patients are to be in Contact Isolation until discharge Never use Alcohol foam or gel for hand hygiene (Alcohol foam and gels do not kill C. diff spores) Always wash hands with soap and water (use friction when washing hands with soap and water to rinse spores down the drain). 8 Toxic Colon from C Difficile 9 Cost of NCI England • Average cost per NCI: 3.000 pounds • Extra days: Urinary tract infections: Pneumonia: Surgical site infections: 6 12 7 Why surveillance? • NCI cause of morbidity and mortality • One third may be preventable • Surveillance = key factor – an infection control measure – overview of the burden and distribution of NCI – allocate preventive resources • Surveillance is cost-efficient!! Infection Control Unit Global Disease Detection & Response Program US Naval Medical Research Unit No.3 Head IC specialists IC training coordinator Epidemiologists M &E specialists Pharmacist Health communication specialist Anthropologist What are Hospital Acquired Infections (HAI’s) • • • • • Blood Stream Infections Ventilator Associated Pneumonia (VAP) Surgical Site Infections (SSI) Urinary Catheter Associated Infection (CAUTI) Multi-drug Resistant Organism (MRO) 13 Healthcare-Associated Infection (HAI) Types HAI Pneumonia Deviceassociated infections (subtypes) Ventilatorassociated (VAP) Urinary tract infection Catheterassociated (CAUTI) Bloodstream infection Central lineassociated (CLABSI) Target of many prevention efforts Picture courtesy S Schrag http://www.lightstalkers.org/images/show/305512 http://www.featurepics.com/FI/Thumb300/20090428/Foley-Bag-1166380.jpg Surgical Site Infection Others Cumulative risk of developing VAP with the duration of mechanical ventilation. • Although length of time with an endotracheal tube in place increases the risk of nosocomial pneumonia, the greatest risk is during the first 2 weeks of intubation. • Nearly all intubated children will have colonization of their endotracheal tube with hospital-acquired organisms by a mean of 5 days. The most common organisms isolated in the developing countries • Enterobacteriaceae spp. (26%) • Pseudomonas aeruginosa (26%), with 60% resistant to fluoroquinolones • S. aureus (22%), with 84% methicillin resistant • Acinetobacter spp. (20%) • The bacterial organisms identified most often are gram-negative bacilli, with P. aeruginosa being the most common species identified in PICUs. • The second most common bacterial etiology of pediatric nosocomial pneumonia is the gram-positive organisms. The frequently isolated bacteria are S. aureus and coagulase-negative staphylococci. • S. epidermidis nosocomial pneumonia is a common cause in the NICU population and is typically the result of hematogenous spread. • Anaerobic nosocomial pneumonia is rare in the pediatric population but accounts for 23% of nosocomial pneumonias in adults. Ventilator associated pneumonia Ventilator-associated pneumonia remain important causes of morbidity and mortality despite advances in antibiotics therapy, better supportive care modalities, and use of a wide-range of preventive measures Ventilator associated pneumonia The exact incidence of VAP is 6 to 20 fold greater than in Non-Ventilated patients. VAP is associated with a higher crude mortality than other hospital-acquired infections (Level II). How does the lung get infected ? Sources of pathogens for VAP include The environment (air, water, equipment,), and Transfer of microorganisms between the patient and staff or other patients (Level II) A number of host- and treatment-related factors, Severity of the patient’s underlying disease, Prior surgery, Exposure to antibiotics Exposure to invasive respiratory devices and equipment. (Level II). How does the lung get infected ? Aspiration of oropharyngeal pathogens, or leakage of secretions around the endotracheal tube are the primary routes of bacterial entry into the lower respiratory tract (Level II) How does the lung get infected ? Hematogenous spread from infected intravenous Catheters. Bacterial translocation from the gastrointestinal tract lumen are uncommon pathogenic mechanisms (Level II) How does the lung get infected ? Infected bio-film in the endotracheal tube, with subsequent embolization to distal airways, may be important in the pathogenesis of VAP (Level III) The sinuses may be potential reservoirs of nosocomial pathogens but their contribution is controversial, (Level II) Things that DO NOT prevent VAP Chest physiotherapy Early tracheostomy. Change of ventilatory circuits Change of HME filters In line suctioning Prophylactic antibiotics Risk factors for Ventilator-Associated Pneumonia (VAP) Patient • Age • Burns • Coma • Lung disease • Immunosuppression • Malnutrition • Blunt trauma Devices • Invasive ventilation • Duration of invasive ventilation • Reintubation • Medication • Prior antiobiotic treatment • Sedation VAP Prevention 1. Hand hygiene before and after patient contact, preferably using alcohol-based handrubbing 2. Avoid endotracheal intubation if possible 3. Use of oral, rather than nasal, endotracheal tubes 4. Minimize the duration of mechanical ventilation 5. Promote tracheostomy when ventilation is needed for a longer term 6. Glove and gown use for endotracheal tube manip VAP Prevention (con’t) 7. Avoid non-essential tracheal suction 8. Oral hygiene with chlorhexidine 9. Backrest elevation 30-45o 10. Maintain tracheal tube cuff pressures (>20) to prevent regurgitation from the stomach 11. Avoid gastric overdistension 12. Promote enteral feeding 13. Careful blood sugar control in patients with diabetes Is there a role for oral antiseptics ? • Oral chlorhexidine application reduces VAP in one study but not for general use – I ATS Guidelines 2005 Catheter Associated Urinary Tract Infections (CAUTI) • Urinary Catheter Associated Infections are defined as an infection occurring 48 hours after insertion of a urinary catheter, signs and symptoms of infection (fever, pain, frequency, urgency, increased white count, etc.) and a positive urine culture of 100,000CFU/ml with no more than 2 species of bacteria. 31 Prevention of Catheter-Associated Urinary Tract Infection (CA-UTI) Two main principles Avoid unnecessary catheterization Limit the duration of catheterization Indications for the use of indwelling urethral catheters • Indications – – – – – Perioperative use for selected surgical procedures Urine output monitoring in critically ill patients Management of acute urinary retention and urinary obstruction Assistance in pressure ulcer healing for incontinent residents As an exception, at patient request to improve comfort • Urinary incontinence is not an accepted indication for urinary catheterization – 21 to 50 percent of urinary catheters not indicated Lo et al. (2008) Infect Control Hosp Epidemiol Suppl 1:S41-50 What you should not do to prevent CAUTI • Do not use (avoid) catheter irrigation (A-I) • Do not use systemic antimicrobials routinely as prophylaxis (A-II) • Do not change catheters routinely (A-III) Lo et al. (2008) Infect Control Hosp Epidemiol Suppl 1:S41-50 Sources of the catheter-associated bloodstream infection Intraluminal from tubes and hubs Hematogenous from distant sites Skin Vein Extraluminal from skin Multimodal intervention strategies to reduce catheter-associated bloodstream infections: - Hand hygiene - Maximal sterile barrier precaution at insertion - Skin antisepsis with alcohol-based chlorhexidinecontaining products - Subclavian access as the preferred insertion site - Daily review of line necessity - Standardized catheter care using a non-touch technique - Respecting the recommendations for dressing change Eggimann P. Lancet 2000; 35: 290 Pronovost P. N Engl J Med 2006; 355: 26 Zingg W. Crit Care Med 2009; 37: 2167 GENERAL PRINCIPLES Good general ward hygiene: - No overcrowding - Good ventilation - Regular removal of dust - Wound dressing early in day - Disposable equipment HAND WASHING most important Before and after patient contact before invasive procedures Many Personnel Don’t Realize When They Have Germs on Their Hands • Healthcare workers can get 100s to 1000s of bacteria on their hands by doing simple tasks like: – – – – – – pulling patients up in bed taking a blood pressure or pulse touching a patient’s hand rolling patients over in bed touching the patient’s gown or bed sheets touching equipment like bedside rails, overbed tables, IV pumps Casewell MW et al. Br Med J 1977;2:1315 Ojajarvi J J Hyg 1980;85:193 Why Don’t Staff Wash their Hands (Compliance estimated at less than 50%) Why Not? • • • • • • Skin irritation Inaccessible hand washing facilities Wearing gloves Too busy Lack of appropriate staff Being a physician (“Improving Compliance with Hand Hygiene in Hospitals” Didier Pittet. Infection Control and Hospital Epidemiology. Vol. 21 No. 6 Page 381) Why Not? • • • • • Working in high-risk areas Lack of hand hygiene promotion Lack of role model Lack of institutional priority Lack of sanction of non-compliers Successful Promotion • Education • Routine observation & feedback • Engineering controls – Location of hand basins – Possible, easy & convenient – Alcohol-based hand rubs available • Patient education (Improving Compliance with Hand Hygiene in Hospitals. Didier Pittet. Infection Control and Hospital Epidemiology. Vol. 21 No. 6 Page 381) Hand hygiene is the simplest, most effective measure for preventing hospital-acquired infections. Any Questions??? • Thank you for not asking!!!