* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download From Bench to Bedside: Clinical Application of Genomic Information
Survey
Document related concepts
Transcript
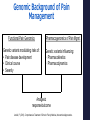
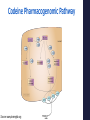
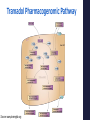
Precision Healthcare: Pharmacogenomics & Pain Management Mitchell Knisely, PhD, RN-BC, ACNS-BC Postdoctoral Scholar University of Pittsburgh School of Nursing February 7, 2017 Overview of Presentation • Advances in Genomic Science • Foundations Practice of Pharmacogenomics & Translation to • Pharmacogenomics & Pain Management • Cytochrome P450 Drug Interactions • Implications for Practice “Evidence suggests that wide variations in clinical practice and inadequate tailoring of pain therapies to individuals...not only contribute to poor quality of care for people with pain, but also increase health care costs.” (National Pain Strategy, 2016) Advances in Genomic Science Human Genome Project 1990-2003 • World-wide research effort • Goal of analyzing the structure of human DNA and determining the location of human genes • Draft of the human genome was announced in June 2000 and published in February 2001 “…the most important, most wondrous map ever produced by humankind.” – President Clinton Benefits of Human Genome Project • Molecular Medicine Improve diagnosis of disease Create drugs based on molecular information • Microbial Genomics Rapidly detect & treat pathogens in clinical practice • Risk Assessment Evaluate health risks faced by individuals who are exposed to certain environmental conditions • DNA Identification (Forensics) Match potential suspects with DNA evidence left at crime scene Post-Human Genome Project Era 2003 - present Collins et al. Nature. 2003;422(6934):835-47. The International Genome Sample Resource: http://www.internationalgenome.org/about Genetic Information Nondiscrimination Act (GINA) • Provides minimum protection against genetic discrimination. • 2 main protections: Total restriction on the use of genetic health information to make employment decisions. Prohibition on making genetic information a requirement for attaining health insurance. Precision Medicine Initiative ® • Announced by President Obama at 2015 State of the Union Address. • The Precision Medicine Initiative® will generate the scientific evidence needed to move the concept of precision medicine into clinical practice. • Precision medicine (health): an emerging approach for disease prevention and treatment that takes into account people’s individual variations in genes, environment, and lifestyle. Precision Health & Pharmacogenomics • Identify genetic or protein biomarkers to predict drug response and side effects. • Identifying the right drug, for the right patient, at the right time, at the most optimal dose. Foundations of Pharmacogenomics & Translation to Clinical Practice “Current” Medical Paradigm “One-size-fits all approach” What is Pharmacogenomics? • Study of genomic variations associated with drug response. • Understand how genetic variation contributes to variability in drug disposition, response, & toxicity. Pharmacogenomics in Practice • 65-90% of adult patients is prescribed 1 or more pharmacogenetically actionable variants. • Lack of efficacy and adverse drug effects r/t interpatient variability = poor patient outcomes and increased healthcare costs • Pharmacogenomic information is clinically actionable – not necessarily “stigmatizing.” What are genetic variations? • A genetic variant is a difference in the DNA sequence compared with a reference sequence. Polymorphism: A genetic variant that is common (≥ 1% in the population). Mutation: A genetic variant that is rare (< 1% in the population. Allele: one of two or more versions of a gene. • Most common type of genetic variation is a single nucleotide polymorphism (SNP). • SNPs can alter a person’s ability to metabolize certain drugs. Factors Affecting Pharmacokinetics & Pharmacodynamics of Drugs Pharmacokinetics Prescribed dosing regimen Pharmacodynamics Drug effects Drug at site of action • Compliance • Dosing & medication errors • Absorption • Tissue & body fluid mass & volume • Drug interactions • Elimination • Drug metabolism • • • • Drug receptor status Genetic factors Drug interactions Tolerance Spruill et al. (2014). Clinical Pharmacokinetics. Bethesda, MD: ASHP. Pharmacogenetic Classification of Genes Drug-transporter pharmacogenetics: • Genes that code for membrane transporters that move drugs either into or out of cells. Drug-target pharmacogenetics: • Genes that code for the direct target of the drug. Pharmacogenetic Classification of Genes Drug-metabolizing pharmacogenetics: • Includes variations in genes that are involved in the metabolism of the drug. • Genes include those that code for metabolizing enzymes that may either: Activate an inactive PRODRUG into an active agent; or Inactivate an ACTIVE DRUG to an inactive metabolite. Genetic Variants’ Clinical Impact on Drug Metabolism Gain-of-function Mutation Wild Type Allele Loss-of-Function Mutation ↑ drug-metabolizing enzyme activity Normal drugmetabolizing enzyme activity No or ↓ drugmetabolizing enzyme activity Active Drug Little or No Active Drug Appropriate Dose ↑ Active Drug Exposure Pro Drug ↑ Active Drug Exposure Appropriate Dose Little or No Active Drug Brazeau, D. (2015). Basics of pharmacogenomics. In D. Lea, D. Cheek, D. Brazeau, & G. Brazeau (eds.), Mastering Pharmacogenomics. Indianapolis, IN: STTI. Drug Metabolizing Phenotypes Phenotype Clinical Interpretation Ultra-rapid metabolizers (UM) Increased risk for toxicity Extensive metabolizers (EM) Should be able to achieve therapeutic effects with normal dosing Intermediate metabolizers (IM) May show reduced effects with normal dosing Poor metabolizers (PM) Lack of therapeutic effects may be observed Translating Pharmacogenetic Testing to Clinical Practice Mitigate/Cure Pharmacogenetic Test Administer drug Genotype Phenotype Adverse drug effect Select alternative drug/adjust dosing Pharmacogenomic Practice Resources U.S. Food & Drug Administration (FDA): > 170 drugs have pharmacogenomic information on drug labels. Source: http://www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics/ucm083378.htm Pharmacogenomic Practice Resources • PharmGKB: www.PharmGKB.org Pharmacogenomic Practice Resources Clinical PGx Implementation Consortium (CPIC): CPIC guidelines help clinicians understand HOW available genetic test results should be used to optimize drug therapy. Not WHETHER tests should be ordered. 36 medications with CPIC Guidelines Additional guidelines from the Dutch Working Group & other professional organizations. Elements of CPIC Guidelines • Introduction • Focused Literature Review • Gene: • Background Genetic Test Interpretation Available Genetic Test Options Incidental findings Other considerations Drug(s): Background Linking genetic variability to variability in drug-related phenotypes Dosage Recommendations Table 2. Recommended Dosing of ____ [drug/s] by ____ [gene] phenotype Strength of recommendations grading system Recommendations for Incidental Findings Other considerations • Potential Benefits and Risks for the Patient • Caveats: Appropriate Use and/or Potential Misuse of Genetic Tests Source: www.pharmgkb.org/page/cpic Pharmacogenomic Practice Resources NCBI – Genetic Testing Registry (GTR): Access to other resources on NCBI: www.ncbi.nlm.nih.gov/guide/genes-expression/. Pharmacogenomics & Pain Management Benefits of Pharmacogenomics in Pain Management • Improved • Ability drug safety. to optimize individual therapies. Drug Safety - Codeine http://www.fda.gov/Drugs/DrugSafety/ucm339112.htm Genomic Background of Pain Management Functional Pain Genomics Genetic variants modulating risks of: • Pain disease development • Clinical course • Severity Pharmacogenomics of Pain Mgmt. Genetic variants influencing: • Pharmacokinetics • Pharmacodynamics Analgesic response/outcome Janicki, P. (2013). Comprehensive Treatment of Chronic Pain by Medical, Interventional Approaches. Genetic Factors Influencing Analgesic Response • Pharmacokinetic Factors Drug-metabolizing enzymes CYP2D6, CYP3A4 Drug elimination UGT2B7 Drug transporters ABCB1, MDR1 • Pharmacodynamic Drug receptors OPRM1, OPRK1 Signal transduction COMT, STAT6 Factors Opioid Signaling Pathway Laugsand et al. European Journal of Cancer. 2011;47:1682-1691. Well-Known Pharmacogenomic Associations: Analgesics Analgesic Genes Dosing Guideline Acetaminophen CYP1A2, CYP2D6, CYP2E1, CYP3A4 None Celecoxib CYP2C9, CYP2D6 None Codeine* CYP2D6, CYP3A4, UGT2B4, UGT2B7 Fentanyl CYP3A4 None Oxycodone CYP2D6 DPWG Tramadol* CYP1A2, CYP2D6, CYP2E1, CYP3A4 DPWG Legend: * = PGx Info on FDA label CPIC: Clinical Pharmacogenetics Implementation Consortium DPWG: Royal Dutch Assoc. for the Advancement of Pharmacy – PGx Working Group PRO: other professional society CPIC, DPWG, PRO Pharmacogenomic Associations: Analgesics Analgesic Genes Morphine UGT2B7, ABCB1, COMT, OPRM1, CGH1 Hydrocodone CYP2D6, ABCB1, COMT Methadone CYP2D6, UGT2B7, ABCB1, OPRM1 Ibuprofen CYP2C9 Diclofenac CYP2C9 Naproxen CYP2C9 Pharmacogenomic Associations: Adjuvants Drug Class Actionable Genes Dosing Guideline CYP2D6, CYP2C19 CPIC CYP2C19 CPIC HLA-B CPIC Tricyclic Antidepressants (TCAs) (e.g., amitriptyline, doxepin, desipramine, imipramine, nortriptyline) Selective Serotonin Reuptake Inhibitors (SSRIs) (e.g., citalopram, escitalopram) Anticonvulsants (e.g., carbamazepine) Codeine Pharmacogenomic Pathway Source: www.pharmgkb.org CYP2D6 Codeine Metabolism Phenotypes Crews et al. Clin Pharmacol & Ther. 2014;95(4):376-382. Codeine CPIC Guideline Crews et al. Clin Pharmacol & Ther. 2014;95(4):376-382. Tramadol Pharmacogenomic Pathway Source: www.pharmgkb.org Tramadol Dosing Guideline Swen et al. Clin Pharmacol & Ther. 2011;89(5):662-673. Oxycodone Dosing Guideline Swen et al. Clin Pharmacol & Ther. 2011;89(5):662-673. Cytochrome P450 Drug Interactions Drug Interactions • Drug-drug interactions (DDI): Occurs when 2 or more drugs interact in such a way that the effectiveness and/or toxicity of one of those agents is affected. • Drug-gene interactions (DGI): Occurs when genotype (e.g., CYP2D6 poor metabolizer) affects the patient’s ability to clear a drug. • Drug-drug-gene interactions (DDGI): Occurs when genotype and another drug in patient’s regimen (e.g., CYP2D6 inhibitor) affect a patient’s ability to clear a drug. Verbeurgt et al. Pharmacogenomics. 2014;5(5):655-65. Drug Interactions Classification of Medications in CYP450 DDGI: Substrates: drugs that are metabolized as substrates by the enzyme Inhibitors: drugs that prevent the enzyme from metabolizing the substrates Activators (inducer): drugs that increase the enzyme's ability to metabolize the substrates P450 Drug-Drug-Gene Interactions Substrates 2D6 Inhibitors Inducers codeine tramadol Oxycodone bupropion cinacalcet fluoxetine paroxetine quinidine diphenhydramine dexamethasone rifampin TCAs duloxetine sertraline terbinafine amiodarone Cimetidine Strong inhibitor: a > 5-fold increase in the plasma AUC values or more than 80% decrease in clearance. Moderate inhibitor: a > 2-fold increase in the plasma AUC values or 50-80% decrease in clearance. Weak inhibitor: a > 1.25-fold but < 2-fold increase in the plasma AUC values or 20-50% decrease in clearance. Complete Flockhart Table™ at http://medicine.iupui.edu/clinpharm/ddis/main-table. Implications for Pain Management Practice Clinical Implications Patient assessment – Understand relationship between genetics & health. Link medication history, medication regimen, and drug responses. Identify at risk individuals who may have altered drug effects. Assist in identifying the most optimal analgesic medication. Patient education – Interpreting genetic test results. (Case study) Provide patients with information & resources for informed decision making. Self-monitoring for drug effectiveness & adverse effects. Referrals – Facilitate referrals for specialized genetic & genomic services. Clinical Implications Implementing PGx Testing Into Practice: Pharmacogenomic Alert • Evaluate & implement best practices Strong Risk of Therapeutic Failure* CYP2D6 *4/*4 (Poor Metabolizer) Patient prescribed codeine Implications: Greatly reduced morphine formation following codeine administration, leading to insufficient pain relief Pharma Management Recommendation: • Integration with electronic health record Avoid codeine use due to lack of efficacy. Consider alternative analgesics such as morphine or a non-opiod. Consider avoiding tramadol. *CPIC Dosing Guideline for codeine and CYP2D6: www.pharmgkb.org * Clinical Pharmacogenetics Implementation Consortium (CPIC) CPIC guidelines reflect expert consensus based on clinical evidence and peer-reviewed literature available at the time they are written and are intended only to assist clinicians in decision making P Request Pharmacogenetic Consult No thanks, continue with order Levy et al. CPT. 2014; 93(3): 307-309 Change Rx Clinical Implications • Cost of testing Ranges from $250-500 • Insurance coverage Most insurance plans consider vast majority of PGx testing “experimental” Medicare's "Coverage with Evidence Development" policy may cover a pharmacogenetic test if a patient has "appropriate" indications • Ethical considerations Online Pharmacogenomic Resources For Clinicians • The Pharmacogenomics Knowledgebase (PharmGKB): https://www.pharmgkb.org/ • Clinical Pharmacogenomics Implementation Consortium (CPIC): https://cpicpgx.org/ • Flockhart Table of CYP450 Drug Interactions: http://medicine.iupui.edu/CLINPHARM/DDIS • FDA Table of PGx Biomarkers in Drug Labeling: http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm • Implementing Genomics in Practice (IGNITE): https://ignite-genomics.org/ • My Drug Genome: http://www.mydruggenome.org/overview.php • Genetics/Genomics Competency Center (G2C2): http://genomicseducation.net/ Contact Information: Mitchell Knisely, PhD, RN-BC, ACNS-BC [email protected]