* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download AP Notes Chapter 11
Survey
Document related concepts
Transcript
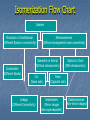
AP Notes Chapter 22 Transition Metals Their Coordination Compounds & Isomers Transition Elements Metals capable of taking on multiple oxidation numbers. Exist in 3 series 1st the 3d 2nd the 4d and the 3rd 5d The 2nd and 3rd series are almost identical in radii size due to the 4f and 5f orbitals, the addition of these 14 protons and little shielding effect cause the similarity in size this is called the lanthanide contraction. Transition Elements Atomic Radii decrease from left to right, with a slight increase at the right end. As Nuclear charge increases the s outer electrons are pulled closer initially making the atom smaller, near the end increased electron repulsion causes the slight increase. Coordination Compounds or complex ions Usually consist of a transition metal and the attraction of a unit called a Ligand with a coordinate covalent bond. They take on a 3-D shape, those shapes or geometries use the naming methods you already know….and then add to them Common coordination numbers are 2,4,and 6 they are associated with geometries linear, tetrahedral, square planar and octahedral. Coordination Compounds Linear – [CuCl2]- , [Ag(NH3)2]+ Tetrahedral – [CoCl4]2- , [NiCl4]2[Ni(CO)4] , [Zn(NH3)4]2+ Square Planar – [Ni(CN)4]2- Octahedral – Fe(H2O)6]2+ , [Co(NH3)6]3+ Coordination Compounds Monodentate - Ligands with one lone pair of electrons to donate Polydentate – Ligands that have more than one lone pair to danate, often on different atoms (one bite) Chelate ring – a polydentate ligand that coordinate bonds to the same atom (two bites) Transition Elements Melting Points increase toward the center of each series where the d orbital is about half-filled, where the largest number of unpaired electrons exist. These metals can be isolated or extracted from ore by processes we call metallurgy Pyrometallurgy – involves high temperatures Hydrometallurgy – involves aqueous solutions Isomerization Flow Chart Isomers Structural or Constitutional (Different Bonds or connectivity) Coordination (Different Bonds) Stereoisomerism (Different arrangement same connectivity) Geometric or Achiral (Without stereocenters) Cis(Same side) Linkage (Different Connectivity) Optical or Chiral (With stereocenters) Trans(Opposite side) Enantiomers (Mirror images Non-superimposible) Diastereoisomer (Non-mirror images) Constitutional isomers: compounds that have the same molecular formula but different structural formulas (connected differently) CH3 CH2 CH2 CH3 Bu tane (bp -0.5°C) CH3 CH3 CHCH3 2-Methylp ropan e (bp -11.6°C) Cis- or Trans- isomers: because of restricted rotation about a carbon-carbon double bond, an alkene with two different groups on each carbon of the double bond shows cistrans isomerism H H H C C H3 C CH3 C C CH3 cis -2-Butene mp -139°C, b p 4°C H3 C H t rans-2-Butene mp -106°C, bp 1°C Crystal Field Theory All the d orbitals are equal in energy they are said to be degenerate Atomic bond theory and molecular orbital theory cannot explain many complexes and their behavior or bonding The electrostatic field or ligand field exists when the atom or ion is approached by a legand. Electrons lose degeneracy and are pushed into higher and lower spins split by energy Crystal Field Theory Fe2+ has d6 electron configuration Fe2+ [Fe(H2O)6]2+ [Fe(CN)6]4- weak field strong field high spin low spin DE Colors & Coordination Componds Transition metal complexes absorb light causing electrons to move from low to high energy levels. The energy difference corresponds to a frequency in the visible portion of the spectrum … they appeared colored Electron transition in the d orbital is responsible for this color Changes in the splitting of the d orbital changes the observed color Ligands ordered in ability to split d i.e. how strong the ligands are, ordering = spectrochemical series