* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download IR Spectroscopy
Spectral density wikipedia , lookup
Homoaromaticity wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Aromaticity wikipedia , lookup
Transition state theory wikipedia , lookup
Chemical bond wikipedia , lookup
Photoelectric effect wikipedia , lookup
Heat transfer physics wikipedia , lookup
Thermal radiation wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
Franck–Condon principle wikipedia , lookup
Two-dimensional nuclear magnetic resonance spectroscopy wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Mössbauer spectroscopy wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
Rotational spectroscopy wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
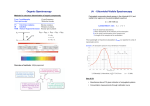
Determining the Structure of an Organic Compound The analysis of the outcome of a reaction requires that we know the full structure of the products as well as the reactants In the 19th and early 20th centuries, structures were determined by synthesis and chemical degradation that related compounds to each other. Physical methods now permit structures to be determined directly. We will examine: infrared (IR) spectroscopy nuclear magnetic resonance spectroscopy (NMR) 1 Principles of Spectroscopy The relationship between energy of light E, and its frequency, is given by the equation: E=h The equation says that there is a direct relationship between the frequency of light and its energy: the higher the frequency, the higher the energy. The proportionality constant between energy and frequency is known as Plank’s constant, h. 2 Principles of Spectroscopy Also, = c / , where c is the speed of light and is the wavelength of light. Therefore, E = h c / This equation tells us that the shorter the wavelength of light, the higher its energy. 3 Principles of Spectroscopy Molecules can exist at various energy levels. For example, The bonds in a given molecule may stretch, bend, or rotate. electrons may move from one orbital to another. These processes are quantized; that is, bonds may stretch, bend or rotate only with certain frequencies, and electrons may jump between orbitals with well defined energy differences. It is these energy differences that we measure by various types of spectroscopy. 4 Spectroscopy of the Electromagnetic Spectrum Radiant energy is proportional to its frequency (cycles/s = Hz) as a wave (Amplitude is its height) Different types are classified by frequency or wavelength ranges 5 Infrared Spectroscopy of Organic Molecules All covalent compounds absorb various frequencies of electromagnetic radiation in the infrared region of the electromagnetic spectrum IR energy in a spectrum is usually measured as wave number (cm-1), the inverse of wavelength and proportional to frequency. The main reason to use wavenumbers as units is that they are directly proportional to energy (a higher wavenumber corresponds to higher energy). In terms of wavenumbers, the IR extends from 4000 to 400cm-1. 6 The infrared absorption process Molecules are excited to a higher energy state when they absorb infrared (IR) radiation. The absorption of IR radiation is a quantized process. The absorption of IR radiation corresponds to energy changes in the order of 8 – 40 KJ/mol (1.9 – 9.5 KCal/mol). Radiation in this energy range corresponds to the stretching and bending vibrational frequencies of the bonds in most covalent molecules. 7 The infrared absorption process When a match occurs between the frequencies of the IR radiation and the natural vibrational frequencies of the molecule, energy is absorbed by the molecule. The absorbed energy serves to increase the amplitude of the vibrational motions of the bonds in the molecule. 8 The infrared absorption process All the bonds in a molecule are not capable of absorbing IR energy, even, if the frequency of the radiation exactly matches that of the bond motion. Only those bonds that have a dipole moment that changes as a function of time are capable of absorbing the IR radiation. Thus, a symmetric bond which has identical or nearly identical groups on each end will not absorb in the infrared. 9 The infrared absorption process A symmetric bond which has identical or nearly identical groups on each end will not absorb in the infrared. 10 Infrared Energy Modes Stretching and bending vibrational motions (modes) that are infrared active give rise to absorptions. Asymmetric stretching vibrations occur at higher frequencies than symmetric stretching vibrations. Stretching vibrations occur at higher frequencies than bending vibrations. Bending vibrations : 1) In-plane a) Scissoring b) Rocking 2) Out-of-plane a) Wagging b) Twisting 11 Gas phase IR spectrum of formaldehyde 12 Overtone, combination and difference bands, and Fermi resonance Apart from fundamental absorptions due to excitation from ground state to the lowest-energy excited state, the spectrum is usually complicated by the presence of weak overtone, combination, and difference bands. Overtone Bands Excitation from ground state to higher energy states, which corresponds integral multiples of the frequency of the fundamental (). Weak overtone bands could occur at 2, 3, …. Combination bands Two vibrational frequencies (1 and 2) in a molecule couple to give rise to a new infrared active frequency. This band is the sum of the two interacting bands (combination = 1 + 2). Difference bands A new infrared active frequency arising due to the difference between the two interacting bands (difference = 1 - 2). Fermi resonance When a fundamental vibration couples with an overtone or combination band, the coupled vibration is called Fermi resonance. Fermi resonance is often observed in carbonyl compounds. These additional weak bands can be calculated directly by multiplication, addition, and subtraction processes. 13 Effect of rotational frequencies Rotational frequencies of the whole molecule are not infrared active However, they often couple with the stretching and bending vibrations in the molecule to give additional fine structures to these vibrations. One of the reasons a band is broad rather than sharp in the IR spectrum is rotational coupling. 14 Bond Properties The natural frequency of vibration of a bond is given by the equation: where µ = m1m2/(m1+m2) (termed the 'reduced mass'), and c is the velocity of light. K is a constant that varies from one bond to another. Force constants for triple bonds are three times those of single bonds. Stronger bonds have a large force constant K and vibrate at higher frequencies Bonds between atoms of higher masses vibrate at lower frequencies than bonds between lighter atoms. 15 Bond Properties Hybridization affects the force constant K. Bonds are stronger in the order sp > sp2 > sp3. Resonance affects the strength and length of a bond and hence its force constant. 16 Interpreting Infrared Spectra Most functional groups absorb at about the same energy and intensity independent of the molecule they are in. Characteristic higher energy IR absorptions can be used to confirm the presence of a functional group in a molecule. IR spectrum has lower energy region characteristic of a molecule as a whole (“fingerprint” region). 17 Regions of the Infrared Spectrum 4000-2500 cm-1 N-H, C- 2000-1500 cm-1 double H, O-H (stretching) 3300-3600 N-H, O-H 3000 C-H 2500-2000 cm-1 CC and C N (stretching) bonds (stretching) C=O 1680-1750 C=C 1640-1680 cm-1 Below 1500 cm-1 “fingerprint” region 18 Appearance of IR Spectrum 19 Usefulness of infrared absorption spectroscopy: Five C4H8O isomers 20 Carbonyl Compounds Aldehydes and Ketones For simple aldehydes and ketones, the stretching vibration of the carbonyl group gives rise to a strong and distinctive infrared absorption at 1710 to 1740 cm-1. Since alkyl substituents stabilize the carbocation character of the ionic contributer, ketone carbonyls have slightly lower stretching frequencies, 1715 ± 7 cm-1, compared with aldehydes, 1730 ± 7 cm-1. 21 Carbonyl Compounds - Aldehydes and Ketones Three factors are known to perturb the carbonyl stretching frequency: 1) Conjugation with a double bond or benzene ring lowers the stretching frequency. 22 Carbonyl Compounds Aldehydes and Ketones 2) Incorporation of the carbonyl group in a small ring (5,4, or 3)\ Raises the stretching frequency. 23 Carbonyl Compounds Aldehydes and Ketones 3) Changing an alkyl substituent of a ketone for an electron releasing or withdrawing group. 24 Carbonyl Compounds Aldehydes and Ketones 25 Carbonyl Compounds Aldehydes and Ketones 26 Carbonyl Compounds Aldehydes and Ketones 27 Carbonyl Compounds Aldehydes and Ketones 28 Carboxylic Acids The carboxyl group is associated with two characteristic infrared stretching absorptions which change markedly with hydrogen bonding. Carboxylic acids exist predominantly as hydrogen bonded dimers in condensed phases. The O-H stretching absorption for such dimers is very strong and broad, extending from 2500 to 3300 cm-1. This absorption overlaps the sharper C-H stretching peaks, which may be seen extending beyond the O-H envelope at 2990, 2950 and 2870 cm-1. The smaller peaks protruding near 2655 and 2560 are characteristic of the dimer. The carbonyl stretching frequency of the dimer is found near 1710 cm-1, but is increased by 25 cm-1 or more in the monomeric state. 29 Carboxylic Acids 30 Carboxylic Acids 31 Carboxylic Acids 32 Carboxylic Acid Derivatives Acyl Halides Acyl Halides (RCOX) The very reactive acyl halides absorb at frequencies significantly higher than ketones. C=O stretch: X = F, 1860 ± 20 cm-1; X = Cl, 1800 ± 15; X = Br,1800 ± 15 33 Carboxylic Acid Derivatives Acid Anhydride Acid Anhydride, (RCO)2O : The very reactive anhydrides absorb at frequencies significantly higher than ketones. C=O stretch: (2 bands separated by 60 30 cm-1) acyclic, 1750 & 1820 cm-1; 6-membered ring, 1750 & 1820; 5-membered ring, 1785 & 1865. 34 Carboxylic Acid Derivatives Esters & Lactones Esters & Lactones (RCOOR'): C=O stretch: Esters, 1740 10 cm-1; 6 membered lactone, 1740 10 cm-1; 5 membered lactone, 1765 10 cm-1; 4 membered lactone, 1840 5 cm-1 35 Carboxylic Acid Derivatives Amides & Lactams Amides & Lactams (RCONR2): The relatively unreactive amides absorb at lower frequencies. C=O bands : 1° & 2°-amides, 1510 to 1700 cm-1 (2 bands) 3°-amides, 1650± 15 (one band); 6-membered lactams, 1670 ± 10 (one band) 5-membered lactams, 1700 ± 15 ; 4-membered lactams, 1745 ± 15. 36 Alcohols and Phenols The O-H stretching absorption of the hydroxyl group is sensitive to hydrogen bonding. In the gas phase and in dilute CCl4 solution (0.01 M) small to moderate sized alcohols exhibit a sharp absorption in the 3620 to 3670 cm-1 region. In more concentrated solution, or as a pure liquid, hydrogen bonding of the hydroxyl groups to each other occurs, and this lowers and broadens the stretching frequencies of the participating O-H bonds. 37 Amines Primary Amine (1°): Primary aliphatic amines display two well-defined peaks due to asymmetric (higher frequency) and symmetric N-H stretching, separated by 80 to 100 cm-1. Strong in-plane NH2 scissoring absorptions at 1550 to 1650 cm-1, and out-of-plane wagging at 650 to 900 cm-1 (usually broad) are characteristic of 1°-amines. 38 Amines Secondary Amine (2°): Secondary amines exhibit only one absorption near 3420 cm-1. Hydrogen bonding in concentrated liquids shifts these absorptions to lower frequencies by about 100 cm-1. 39 Amines Tertiary Amine (3°): No N-H absorptions. The C-N absorptions are found in the same range, 1200 to 1350 cm-1 (aromatic) and 1000 to 1250 cm-1 (aliphatic) as for 1°-amines. 40 Arenes In addition to the stretching vibrations at 3000 70 cm-1, several sharp, weaker absorptions in the 950 to 1250 range due to in-plane C-H bending vibrations also occur.. These are not diagnostically useful, except for indicating a substituted benzene ring. Weak overtone and combination tone absorptions are found in the 1600-2000 region 41 Arenes Ortho substituted dialkyl arene 42 Arenes Meta substituted dialkyl arene 43 Arenes Para substituted dialkyl arene 44