* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download IR Lecture
Survey
Document related concepts
Transcript
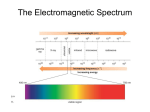
Infrared Spectroscopy I. Infrared Spectroscopy A. Energy Absorption and Vibration 1) IR electromagnetic radiation is just less energetic than visible light 2) This energy is sufficient to cause excitation of vibrational energy levels c λυ 3.0 x 108 m/s 3) Wavelength (l) = 2.5-16.7 x 10-6 m ~ 1 l 600 - 4000 cm 1 1 10 kcal/mol 1 l E h hc l hc h Planck' s Constant 6.626 x 10-34 J s 4) 5) n = wavenumbers. Larger n = higher energy Excitation depends on atomic mass and how tightly they are bound a) Hooke’s Law for 2 masses connected by a spring (m m2 ) ~ k f 1 m1m2 b) c) k = constant f = force constant = bond strength m-term = reduced mass C—H Bond: Reduced Mass = (12+1)/(12x1) = 13/12 = 1.08 C—C Bond: Reduced Mass = (12+12)/(12x12) = 24/144 = 0.167 Many possible absorptions per molecule exist: stretching, bending,… Vibrational modes leading to IR absorptions: B. Using IR in Organic Chemistry 1. Functional Groups have characteristic IR absorptions 2. Fingerprint Region (600-1500 cm-1) is unique for every molecule and lets us match an unknown with a known spectrum Regions of the Infrared Spectrum 4000-2500 cm-1 N-H, C-H, O-H (stretching) A. 3300-3600 N-H, O-H B. 3000 C-H II. 2500-2000 cm-1 CC and C N (stretching) I. 2000-1500 cm-1 double bonds (stretching) A. C=O 1680-1750 B. C=C 1640-1680 cm-1 II. Below 1500 cm-1 “fingerprint” region I. 12.8 Infrared Spectra of Some Common Functional Groups Alkanes, Alkenes, Alkynes I. C-H, C-C, C=C, C C have characteristic peaks A. absence helps rule out C=C or C C 4. IR of Alkenes a. Alkene C—H absorbs at higher energy than alkanes because the force constant is stronger than alkanes (sp2 hybridization) b. Substitution pattern of alkenes give characteristic absorptions i. Terminal alkenes give 910, 990 cm-1 H H C C H ii. R Geminal disubstituted gives 890 cm-1 H R C C H R iii. trans disubstituted gives 970 cm-1 H R C R C H IR: Aromatic Compounds I. Weak C–H stretch at 3030 cm1 II. Weak absorptions 1660 - 2000 cm1 range III. Medium-intensity absorptions 1450 to 1600 cm1 IR: Alcohols and Amines O–H 3400 to 3650 cm1 A. Usually broad and intense II. N–H 3300 to 3500 cm1 A. Sharper and less intense than an O–H I. IR: Carbonyl Compounds I. II. Strong, sharp C=O peak 1670 to 1780 cm1 Exact absorption characteristic of type of carbonyl compound A. 1730 cm1 in saturated aldehydes B. 1705 cm1 in aldehydes next to double bond or aromatic ring C=O in Ketones I. 1715 cm1 in six-membered ring and acyclic ketones II. 1750 cm1 in 5-membered ring ketones III. 1690 cm1 in ketones next to a double bond or an aromatic ring I. II. 1735 cm1 in saturated esters 1715 cm1 in esters next to aromatic ring or a double bond C=O in Esters