* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download DNA methylation
Survey
Document related concepts
Transcript
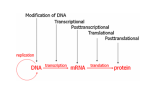
Manifestation of Novel Social Challenges of the European Union in the Teaching Material of Medical Biotechnology Master’s Programmes at the University of Pécs and at the University of Debrecen Identification number: TÁMOP-4.1.2-08/1/A-2009-0011 Manifestation of Novel Social Challenges of the European Union in the Teaching Material of Medical Biotechnology Master’s Programmes at the University of Pécs and at the University of Debrecen Identification number: TÁMOP-4.1.2-08/1/A-2009-0011 Dr. Péter Balogh and Dr. Péter Engelmann Transdifferentiation and regenerative medicine – Lecture 4 EPIGENETIC FACTORS IN TRANSDIFFERENTIATIO N TÁMOP-4.1.2-08/1/A-2009-0011 Epigenetics • Epigenetics is a central molecular mechanism in organism complexity • Epigenetics studies the heritable changes in genome function that occur without a change in DNA sequence. • Even if organisms have the same genotype, depending on environmental changes, they can have different phenotypes that are mediated by epigenetics. • 1942. C. Waddington • Genetic code – epigenetic code / histone code • Main forms: DNA methylation, chromatin TÁMOP-4.1.2-08/1/A-2009-0011 Chromatin remodelling and histone modifications Chromatin changes: • Histons have altered structure or charge (“cis”) • Histons have altered affinity to chromatin associated proteins (“trans”) Histon modifications: postranslational modifications of histon globular domain • Methylation mediated by protein arginine methyltransferase (PRMT) and histone lysine methyltransferases (HKMT) • Acetylation histone acetylases (HAT), and histone deacetylases (HDAC) • Ubiquitination • Sumoylation • Phosphorylation • Citrullination • ADP-ribosylation Epigenetic gene regulation of stem cell genome TÁMOP-4.1.2-08/1/A-2009-0011 DNA repair DNA replication DNA methylation Chromatin remodelling Non-coding RNAs Histone modification Chromatin package Transcription regulatio H2A H3 H3 Methylation Acetylation H4 H4 H2B H2B Phosphorylation Ubiquitylation TÁMOP-4.1.2-08/1/A-2009-0011 DNA methylation • Covalent modifications of cytosine nucleotides of CpG dinucleotides • Majority of methylated CpG dinucleotides are present in heterochromatic region • Promoter CpG islands • DNA methylation is controlled by DNA methyltransferases (Dnmts) – Dnmt1: methylation of hemimethylated CpG sites – Dnmt3a and Dnmt3b: de novo symmetric methylation of DNA during embryonic development and differentiation • Function: methylation in mammals is required for gene silencing (NB. methylation of CpG islands on one X chromosome TÁMOP-4.1.2-08/1/A-2009-0011 Detection procedures of DNA methylation • Affinity purification of methylated DNA • Digestion with methylation-sensitive restriction endonucleases • Bisulfite conversion – Methylation specific PCR – MethyLight (qPCR based approach) – Microarray based approaches – Bisulfite sequencing TÁMOP-4.1.2-08/1/A-2009-0011 DNA methylation in stem cells Transcriptional activation Passive demethylation Transcriptional repression Oct4 Sox2 Klf4 Transcriptional repression DNMT1 Active demethylation Transcriptional repression Transcriptional activation Klf4 Sox2 Oct4 Klf4 Sox2 Oct4 Demethylase Symmetrically methylated DNAHemi methylated DNA TÁMOP-4.1.2-08/1/A-2009-0011 DNA methylation profile of ES cells • Specific methylation profile can be compared between stem cells and adults cells • The pluripotency-associated genes Oct4 and Nanog are largely unmethylated in ESCs and induced pluripotent stem cells (iPSCs), and methylated in differentiated cells • In ES cells, high CpG promoters have low DNA methylation levels, whereas low CpG promoters have relatively high DNA methylation levels TÁMOP-4.1.2-08/1/A-2009-0011 Histone methylation Histone methylation (mono-, di-, tri-) by PRMT and HKMT enzymes can induce ON/OFF signature for gene expression. • Methylation of lysine 4, 36, or 79 on histone 3 (H3K4, H3K36, H3K79), lysine 20 of H4 (H4K20), and lysine 5 of K2B (H2BK5) accelerate gene transcription. • Trimethylation of H3K9, H3K27, or H4K20 represents the inhibition of gene expression. TÁMOP-4.1.2-08/1/A-2009-0011 Histone methylation in stem cells DNA methylation data were examined in conjunction with data on histone modifications: • H3K4me3 (which is generally considered to be an activating mark) and H3K27me3 (a repressive mark), it was found that genes associated with H3K4me3 alone had the lowest levels of promoter methylation (40%) • 47% of the genes with the H3K4/H3K27 “bivalent” mark were methylated • 70% of the genes with H3K27 alone were methylated • 87% of the genes carrying neither histone TÁMOP-4.1.2-08/1/A-2009-0011 Histone acetylation • Acetylation and deacetylation of lysine residue in histone tails is mediated by histone acetyl transferases (HATs) and histone deacetylases (HDACs). • 6 HAT complexes and 18 HDACs have been identified in mammals. • Acetylation brings in a negative charge, acting to neutralize the positive charge on the histones and decreases the interaction of the N termini of histones with the negatively charged phosphate groups of DNA. TÁMOP-4.1.2-08/1/A-2009-0011 Histone acetylation in stem cells I • Histone acetylation status of conserved lysine residues in the amino- terminal of histone H2A, H2B, H3, and H4 contributes to transcriptional regulation. • In general, an increase of histone acetylation by HATs causes remodeling of chromatin from a tightly to a loosely packed configuration (euchromatin), which subsequently leads to transcriptional activation. Conversely, a decrease of histone acetylation by HDACs results in a condensed chromatin structure (heterochromatin) and finally transcriptional silencing TÁMOP-4.1.2-08/1/A-2009-0011 Histone acetylation in stem cells II • Transcription factors Oct4 and Nanog promoters are associated with activating marks such as acetylation of H3 and H4 and H3K4me3 in ES cells. • Adult hippocampal-derived NSCs differentiate predominantly into neurons, at the expense of astrocytes and oligodendrocytes, when treated by the antiepileptic and HDAC inhibitor valproic acid (VPA) in vitro. • VPA-mediated HDAC inhibition upregulates the neuron-specific gene NeuroD, a neurogenic basic helix-loop-helix TF, resulting in the induction and suppression, respectively, of neuronal and glial differentiation. Ubiquitination and sumoylation TÁMOP-4.1.2-08/1/A-2009-0011 Ubiquitin : • is a protein moiety that is covalently attached to proteins via a series of enzymatic steps involving an ubiquitin activating enzyme , a ubiquitin conjugating enzyme and ubiquitin ligase . • Histone H2A was the first protein identified that served as a substrate for ubiquitin. • Specific histon ubiquitination regulate histone methylations. SUMO (small ubiquitin-like modifier) proteins : • covalently attaches to the residues of specific target proteins and alters a number of various functions. • Sumoylation counteracts ubiquitination and subsequent proteosomal degradation via competition with the same lysine residue of substrates. • Sumoylation regulatory mechanism is analogous to Citrullination and phosporylation • TÁMOP-4.1.2-08/1/A-2009-0011 Citrullination or deimination is a posttranslational modification meaning the change of arginine amino acid into citrulline by peptidylarginine deiminases (PADs). • The conversion of arginine into citrulline in histones can have important consequences for the structure and function of proteins, since arginine is positively charged at a neutral pH, whereas citrulline is uncharged which means protein folding changes. • Histon phosphorylation occurs with H2A 139 serine residue in humans, which lead downstream protein target activations. TÁMOP-4.1.2-08/1/A-2009-0011 Polycomb group factors • Polycomb (PcG) proteins are transcriptional regulators control the expression of many genes from embryogenesis til adulthood. • Polycomb group protein family can remodel chromatin such that epigenetic silencing of genes take place. • PcGs are evolutionarily conserved transcriptional repressors, originally described as Hox gene repressor. • PcG proteins form three complexes: Polycomb repressive complex 1 (PRC1), PRC2, and PhoRC. • Two conserved repressor complexes (PRC1 and PRC2) • PRC1 is composed by Cbx, Ring1, Phc, and Bmi1/Mel18PRC2 • PRC2 is composed by EED, SUZ12, EZH2 factors TÁMOP-4.1.2-08/1/A-2009-0011 Polycomb group proteins in stem cells • In ES cells pluripotency depends on the activities of PcG and trithorax (TrxG) complexes. • PRC2 proteins as well as Ring1b or Ring1a regulate the transcription of many genes in ES cells. • PRC2 catalyzes the di and trymethylation of H3K27 motifs. • PRC1 and PRC2 in ES cells targets promoters of >2,000 genes, of which a large subset overlaps with target genes of OCT4, NANOG, and SOX2 transcription factors. TÁMOP-4.1.2-08/1/A-2009-0011 Non-protein coding RNA: Story I mRNA: DNA -------- protein tRNA, rRNA: structural, catalytic decoding function • 1998: Craig Mello, Andrew Fire: non-coding RNA sequences, RNA interference in C. elegans • 2006: Nobel prize TÁMOP-4.1.2-08/1/A-2009-0011 Non-protein coding RNA: Story II • The petal coloration of Petunia was supposed to be changed by introducing the chalcon sythase gene (coding a key enzyme for coloration of petals) for overexpression. • However it resulted a less colored or even total white flowers instead of darker coloration. This supposed to be happened by inhibition of the enzyme activity. Actually, both the endogen and the transgene activitity was reduced in the white flowers. This phenomenon was described as a corepression, but the precise molecular mechanism remained unclear. Non coding RNA, RNA interference TÁMOP-4.1.2-08/1/A-2009-0011 • siRNA(small interfering RNA): 21-22 nt long dsRNA, gene silencing by siRNA, complementer sequences, inhibitory proteins participate in RNAi. • miRNA (microRNA): 19-25 nt long, ssRNA molecules, genomic, evolutionary conserved • tncRNA (tiny non-coded RNA): newly identified in C.elegans, 20-22 nt long genomic RNA, function is unknown. • smRNA (small modulatory RNA): described in 2004 from mice, neuron specific short dsRNA. • PIWI associated small RNA (piRNA): 24-30 nt long Drosophila, mammals, retrotransposons, TÁMOP-4.1.2-08/1/A-2009-0011 miRNA role in stem cells I • Dicer 1 and Dgcr8 enzymes are essential in miRNA biogenesis, stem cells are defective for these enzymes have pluripotency but lower proliferation and differentiation capacity. • miRNAs encoded by Dlk1–Dio3 gene cluster are also candidates for promoting reprogramming because activation of imprinted Dlk1–Dio3 gene cluster is essential for generating fully reprogrammed iPS cells, which are functionally equivalent to ES cells. • miRNAs belonging to the miR-290 and miR- 302 clusters are expressed in both human and mouse ES cells. TÁMOP-4.1.2-08/1/A-2009-0011 miRNA and stem cell differentiation ES/iPS Cells Somatic Cells miR-470 miR-134 inhibitor inhibitor ESCC miRNAs Myc induced miR-92b miRNAs miR-296 ESCC miR-520 miR-470 Myc induced cluster miR-200 miR-145 inhibitor inhibitor miRNAs miRNAs miR-141 miR-296 + miR-520 miR-429 p53 + ? cluster + miR-17-92 cluste miR-134 + miR-92b miR-145 p21 + ? ? ? + ? ? ? + Self-renewal and pluripotency Reprogramming by Sox2, Oct4, Klf4, and c-Myc or Sox2, Oct4, Nanog, and Lin28 let-7 ? + miR-125 inhibitor let-7 inhibitor miR-125 miR-24-1 miR-23b ? miR-21 Inhibitors of miR-24-1, miR-23b, miR-21 + TGF-beta signalling Active Dlk1-Dio3 gene cluster + HDAC inhibitor CpG Inactive Dlk1-Dio3 gene ? miRNAs coded by Dlk1-Dio3 gene cluster Oct4 + ? Dnmt knock-down/Dnmt inhibitors Dnmt 3a and 3b miR-29b mCpG Oct4 TÁMOP-4.1.2-08/1/A-2009-0011 miRNA role in stem cells II • miR-124a is expressed predominantly in neural tissues and has been shown to participate in the in vitro differentiation of NSCs into neurons by mediating degradation of non-neuronal gene transcripts. • Both miR-124 and miR-9 promotes neuronal differentiation, while their down regulation has the opposite effect. • miR-294 exhibited the greatest effect on reprogramming and increased efficiency of iPS cell generation from 0.01–0.05% to 0.4–0.7%. Additionally, miR-294 increased the kinetics of Sox2, Oct4, and Klf4 mediated reprogramming. • Inhibiting the activity of both miR-125 and let-7 miRNAs may result in additional beneficial Cross-talk between genetic and epigenetic regulators in ESCs I TÁMOP-4.1.2-08/1/A-2009-0011 • Transcription factors and epigenetic factors interact on multiple dimensions. • Key pluripotency factors such as Oct4 and Nanog interact physically with epigenetic regulators to maintain ESCs in an undifferentiated state. • Oct4, Sox2 and Nanog co-occupy many of the PRC2 target genes, which are enriched for developmental/differentiation genes. • Through protein interactions, transcription factors such as Oct4 may directly recruit and provide target specificity for repressive PcG proteins such as Ring1b. Cross-talk between genetic and epigenetic regulators in ESCs II TÁMOP-4.1.2-08/1/A-2009-0011 • Transcription factors and epigenetic factors interact to modulate the ESC regulatory network. • Several ESC-specific epigenetic factors are regulated by the core ESC transcription factors. • Oct4 activates the expression of histone demethylases, Jmjd1a and Jmjd2c, which in turn modify H3K9 methylation and regulate the expression of Tcl1 and Nanog. Therapeutical considerations TÁMOP-4.1.2-08/1/A-2009-0011 • Epigenetic changes are reversible upon administration of Dnmt inhibitors and histone deacetylase inhibitors (HDACi) • Dnmt inhibitors such as azacitidine (5-azacytidine), decitabine (5-aza-2`deoxycytidine) has been approved by FDA in cancer treatment. • HDACi such as hydralazine and magnesium valproate are capable to sensitize tumor cells to chemotherapy in patients with solid tumors. • How about cancer stem cells?? TÁMOP-4.1.2-08/1/A-2009-0011 Summary • Life of an individual is not only defined by her/his genome, but also by the numerous epigenomes, with different epigenomes being generated through development. • Epigenomes react to environmental influence including maternal care, diet, exposure to toxins and xenobiotics, and epigenetic responses to environmental stimuli may have long-term consequences, even affecting future generations. • At various level epigenetics affects the stem cell niche, and they build up multiple levels of regulatory circuits in progenitor