* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Early chemical arts
Survey
Document related concepts
Transcript
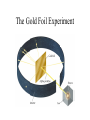
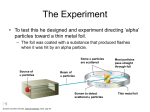
Early chemical arts Chemical arts evolved in the ancient world long before any theories of matter were formulated. Copper Age Axe Iron Age Swords As early as 8,000 years ago, people in the Middle East were smelting copper by heating copper ores with charcoal to high temperatures. The use of gold, bronze, tin, iron, and lead would follow in time. Empedocles • 450 BC • Proposed that matter was composed of four elements: – – – – Earth Air Fire Water Ancient Greek ideas on matter Democritus (c. 460–c. 370 B.C.) proposed that matter was made of discrete indivisible particles, after his teacher pointed out that a beach looks smooth from afar but is really made of discrete grains of sand. He called his particles atomos, meaning "cannot be cut." His ideas were largely ignored until the scientific revolution of the 16th, 17th, and 18th centuries. Aristiotle:Later, Aristotle (c. 384–c. 322 B.C.) popularized the idea that all matter was made of earth, air, water, and fire in varying proportions. According to this notion, one should be able to make gold from other materials by adjusting the ratios of the four elements therein. His ideas influenced alchemy and protochemistry for 2,000 years. Alchemists • First people to perform hands on experiments. • Three main beliefs: – Some elements could be changed into others (such as lead or tin into gold). – They could find a substance that would give them eternal life – They could produce a universal solvent that would dissolve all other substances. Sir Francis Bacon • 1600s • Developed new knowledge about the physical world through use of the scientific method Robert Boyle 1627-1691 • Boyle investigated gasses • Found a law defining the volume of a gas based on pressure and temperature Boyle Re-Defines Elements • Boyle (1627–1691) redefined an element to be a substance that could not be broken down into simple substances and questioned the elemental nature of Aristotle’s foursome. • Boyle recognized that elements could be combined to form compounds. Antoine Lavoisier • Late 1700s • Defined the element as a pure substance that cannot be broken down • Discovered and identified 23 elements • Recognized that mixtures exist. • Classified air as a mixture of oxygen and some other gases. Henry Cavendish • Late 1700s • Experimented by mixing metal with acid, which produced a flammable gas (hydrogen). • He discovered that this gas would burn in oxygen to produce water. John Dalton (early 1800s) • Things they knew by Dalton’s time: – Elements were defined as substances which could not be broken down further by chemical means. (Lavoisier 1775) – There was a law of definite proportions (elements for a given compound always combined in the same ratio) – There was a law of conservation of mass. (In a chemical reaction mass is never created or destroyed. John Dalton • Was born in 1766 • Was a school teacher and later a University Professor • Used pre-existing laws and his own experiments to come up with the first atomic theory • Came up with the first modern atomic theory Dalton’s Atomic Theory 1808 • All matter is made up of small particles called atoms. • Atoms cannot be created or destroyed, or cut into smaller particles • All atoms of the same element are identical in mass and size, but they are different in mass and size from the atoms of other elements. • Compounds are created when atoms of different elements link together in definite proportions Dalton’s Billiard Ball Model • The atom, according to Dalton, was much like a tiny billiard ball. • An element is a substance made up of many very tiny particles (or many tiny billiard balls) which are all identical. • You could physically cut large chunks of element into smaller pieces (like cutting small chips of Iron off an anvil) but you could not chemically break that element apart J.J. Thomson (1904) • Joseph John Thompson was born in 1856 • Was a top student in mathematics and physics • Ended up as head of Physics at Cambridge University in England • Presided over a very productive physics department that produced 7 Nobel prize winners Thomson’s Plum Pudding Model • Thompson reasoned that because the gases in the tube were neutral to start, there must be a balance of charge in an atom • Since he had no way to detect the proton, he reasoned that the atom was much like a plum pudding. • The negative electrons were like plums floating in a pudding of positive charge. • These charges were exactly equal in a neutral atom Ernest Rutherford • Ernest Rutherford was born in 1871 in New Zealand and had 11 brothers and sisters. • Graduated from Canterbury College in Nelson NZ first in his class. • Was awarded a trinity scholarship in 1895 to study at Cambridge under J.J. Thompson. • In 1897 he took up a chair as head of the physics department at the McGill University in Montreal Discovering the Nucleus • In 1911, through the results of his “Gold Foil” experiment, Rutherford discovered the atomic nucleus. • The alpha ( α ) particle is a positively charged, dense particle, equal to the nucleus of a helium atom (+2) • Rutherford fired these particles at an extremely thin sheet of gold foil. The Gold Foil Experiment •Using the Thompson model, Rutherford thought that all the positive alpha particles should pass straight through the foil •In actuality a few of the particles were deflected, while some shot directly back toward the source of the particles! A new Atomic Theory • Rutherford’s deflected alpha particles meant that there must be an extremely massive, tiny positively charged nucleus inside the atom. • It was these positive Nuclei that were deflecting the particles • He proposed that the much lighter electrons rotated around this nucleus. • This became known as the Planetary Model. The Neutron • Protons accounted for only about half the total mass of the gold atom • Rutherford inferred that there must be something similar in mass to the proton, but neutrally charged inside the nucleus • In 1930, the existence of the neutron was proved. • The force of the neutron prevents the repulsion between the protons from pushing the nucleus apart. Neils Bohr • Bohr used the fact that elements, when excited (by heat or electricity), emit characteristic spectra. • For instance copper will glow greenish blue, while aluminum will glow white • This fact had been known for millennia by fireworks makers. Bohr’s Conclusions • After studying the spectra of the simplest elements, Bohr refined the Rutherford model • The Bohr/Rutherford model is still a planetary one, however law’s about how the electrons behave were defined. The Bohr Model • Electrons are found only in very specific energy levels inside the atom. (also known as orbitals or shells) • When excited, the electrons jump to higher energy levels • Electrons may also lose energy and fall to lower energy levels • Photons of certain wavelengths are emitted when the electrons decrease in energy.