* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Option D8 Drug Action HL
Survey
Document related concepts
Transcript
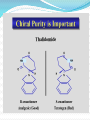
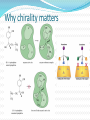
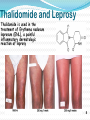
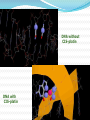
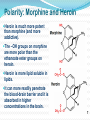
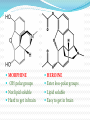
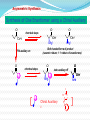
Objectives D.8.1 Describe the importance of GEOMETRICAL ISOMERISM in drug Action D.8.2 Discuss the importance of CHIRALITY in drug action. D.8.3 Explain the importance of beta-lactam ring action of penicillin D.8.4 Explain the increased potency of diamorphine (heroin) compared to morphine. Drug Action The effectiveness of a drug is often related to the chemical structure and polarity of the substance. Factors that affect how a drug reacts include: Chirality 2. Geometrical isomerism 3. Ring strain 4. Polarity 1. 3 Chirality Affects Drug Behavior The presence of an asymmetric or chiral carbon atom in a molecule results in two different optical isomers or ENANTIOMERS. Two different enantiomers can behave in very different ways in the body. The most famous example of this difference was found in with thalidomide. 4 Thalidomide Thalidomide has two optical isomers, one of which is a tranquilizer while the other is a powerful teratogen. Chiral Carbon Originally used to treat morning sickness during pregnancy. Now used to treat some symptoms of Hansen’s disease (Leprosy). The high incidence of fetal deformities has led to increased diligence in approving drugs for use. When new drugs are developed now The pharmacological activity of each optical isomer must be studied 5 separately. Why chirality matters Thalidomide and Leprosy Thalidomide is used in the treatment of Erythema nodosum leprosum (ENL), a painful inflammatory dermatologic reaction of leprosy 8 Geometric Isomers Differ in Behavior Geometric isomerism occurs in both organic and inorganic compounds. Diaminechloroplatnium (II) is an inorganic complex that as been used to treat certain types of ovarian and testicular cancers. Diaminechloroplatnium (II) exists in both cis and trans isomers. 9 Cis and Transplatin The structures of the cis and trans forms are shown below: Cis-platin is an effective anticancer drug, while Trans-platin is not effective at all. 10 Cisplatin as an Anti-cancer Drug Cisplatin can diffuse through a cancer cell membrane. In the cell it exchanges a chloride ion for a water molecule forming a complex ion. This complex ion binds to the cancer cell DNA preventing it from replicating correctly. The cis-platin form is just the right size to bind to the guanine bases on the DNA. 11 DNA without CIS-platin DNA with CIS-platin Ring Strain The action of beta lactams in antibacterial drugs such as the penicillins is an example of the effect of ring strain as a drug mechanism. The general structure of penicillin The four member ring of the betal lactam contains a carbon that is sp2 hybridized and a nitrogen atom and 2 carbon atoms that are sp3 hybridized. The restrictions of the ring prevent the atoms from assuming the normal bond angles of 120o and 109.5o respectively. 13 Ring Strain and Drug Activity The amide group in the ring is more reactive due to the strained ring. The structure of the beta lactam is similar to the structures of cysteine and valine. The beta lactam binds to the enzyme that synthesizes the cell wall in bacteria blocking its action. As a result the bacteria rupture and break and cannot reproduce. Note the similarities in structure to the beta lactam. 14 Polarity Polarity and Drug Behavior The difference in polarity between molecules affects their behavior in the body. The polarity of a molecule affects: 1. its ability to dissolve in lipids 2. its ability to pass through the lipid membranes 3. the degree to which it may bind to an active site on an enzyme or protein. 16 Polarity: Morphine and Heroin Heroin is much more potent than morphine (and more addictive). The –OH groups on morphine are more polar than the ethanoate ester groups on heroin. Heroin is more lipid soluble in lipids. It can more readily penetrate the blood-brain barrier and it is absorbed in higher concentrations in the brain. 17 MORPHINE HEROINE -OH polar groups Ester less-polar groups Not lipid soluble Lipid soluble Hard to get in brain Easy to get in brain Modifying polarity Amine + HCl => hydrochloride salt (ionic, more soluble) Prozac Modifying polarity Carb. Acid + NaOH => Sodium salt (ionic, more soluble) Aspirin Thalidomide Read the three articles on Thalidomide in class today. Write a 2-3 page integrated paper in which you discuss adequately the following; 1. What is thalidomide? When did the drug first appear on the market? What was its original purpose 2. What was there about thalidomide that makes the drug hazardous? Explain and describe the tragic results that resulted from thalidomide use. 3. Thalidomide has two enantiomers. Explain the differences. Are both isomers teratogens? 4. What kinds of problems with drug regulations allowed thalidomide to be marketed in Europe but not in the USA? 5. What kinds of testing should be required before a drug is approved for use? In what respect is animal testing important in this respect? 6. Recently thalidomide has been used to treat other conditions. What are these conditions? What are the benefits and potential risks Drug Design Part 2 22 What is Combinatorial Chemistry? Is an approach that provides efficient synthesis of a large collection of molecules Screening of libraries of related compounds to isolate the molecule of desirable property Used in both academia and industries to generate huge libraries of compounds that have important biological properties Combinatorial Chemistry Drug companies have developed libraries of compounds which have been screened for drug activity. With a given core molecule or pharmacore, and a large number of substituents, researchers use computers to enumerate a large number of structural possibilities. This virtual library may consist of thousands, or even millions of 'virtual' compounds. Researchers select a subset of the 'virtual library' for actual synthesis, based upon various calculations and criteria. 24 Combinatorial Chemistry An example of a pharmacore and a reactant system. By examining multiple possibilities pharmaceutical chemists can evaluate the medical efficacy of various molecules for medicinal value. 25 Combinatorial Chemistry The process was originally developed for polypeptide synthesis with amino acids. The starting material or pharmacore is covalently bonded to small polystyrene resin beads. The beads are reacted with various groups in successive steps. The beads are separated from the reaction mixture and then undergo preliminary screening for drug activity. This is usually done by measuring how the substance affects enzymes or how it may bind to receptor cells. 26 Solid Phase Library In 1991s, Houghten & Lam: synthesis of a huge peptide library 20 amino acids Solid-phase synthesis 202 = 400 dipeptides DNA: fully automatic (solution) peptide 203 = 8000 tripeptides 204 = 160,000 tetrapeptides carbohydrate small molecule (drug-like) ln 1992, Jon Ellman: synthesis of non-peptide drug-like molecules by solid phase synthesis Combinatorial Chemistry A combinatorial scheme for amino acids 28 Parallel Synthesis Alternative to combinatorial approach, Solid state organic. Preparation of a highly reactive intermediate. Preparation of individual compounds simultaneously with various reagents in separate microcells without mixing intermediates during synthesis. 29 Parallel synthesis On a teflon reaction block Large number of wells Add different parts at each step Common conditions 12-well reaction block Add Scaffold to each well S S S S S S S S S S S S Wells after Addition of first reagent SA SB SC SD SA SB SC SD SA SB SC SD There are now twelve different products SA1 SB1 SC1 SD1 SA2 SB2 SC2 SD2 SA3 SB3 SC3 SD3 Drug Polarity Modifications Many compounds that are of pharmacological importance are large complex organic molecules that are not very polar They are largely insoluble in water. Their ionic salts, either as sodium salts or hydrochloride salts are used to make them more soluble. Aspirin or acetyl salicyclic acid is converted to the sodium salt. Sertraline is an amine compound that is converted to a hydrochloride salt to make it more soluble. 35 Chiral Auxillaries Traditional synthesis of optically active compounds results in a racemic mixture with equal amounts of each enantiomer. Only one of the enantiomers has pharmacological value. (i.e. thalidomide). Separating enantiomers from racemic mixtures is often difficult and complicated. The use of chiral auxilliaries makes it possible to synthesize only one of the two enantiomers. A chiral auxilliary is a chiral molecule that is attached to the starting material during a synthesis that creates the appropriate stereo-chemical environment so that only one enantiomer is produced. 36 Asymmetric Synthesis Synthesis of One Enantiomer using a Chiral Auxiliary O O O chemical steps OH NH 2 OH Put auxiliary on OH NH 2 Bothhandedforms of product (racemic mixture; 1:1mixtureof enantiomers) O O chemical steps O takeauxiliary off OH NH 2 NH 2 O [ HN Chiral Auxiliary : O ] Synthesis with Chiral Auxilliaries A chiral auxiliary is a molecule that is temporarily incorporated into an organic synthesis. Its asymmetry allows the formation of a chiral intermediate followed by selective formation of one of two stereoisomers depending on the reagent and/or reaction 38 conditions. Taxol The anti-cancer drug TAXOL is found in the Pacific Yew tree, but there is not a sufficient supply to meet demand. SinceTaxol is a very chiral molecule, one possibility is to make it synthetically. The potential synthesis is very complicated and would require using several chiral auxilliaries.. 40 Computer aided drug design