* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Atomic Structures Study Guide
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Photoelectric effect wikipedia , lookup
Bremsstrahlung wikipedia , lookup
Grand Unified Theory wikipedia , lookup
Compact Muon Solenoid wikipedia , lookup
Electric charge wikipedia , lookup
Standard Model wikipedia , lookup
Electron scattering wikipedia , lookup
Elementary particle wikipedia , lookup
Introduction to quantum mechanics wikipedia , lookup
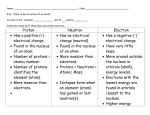
Name____________________ Due Date__________ Date_______________ Period__________ Atomic Structure Study Guide 1). Fill in the names of the atomic particles that show the property described. a. ____________________ -positively charged particle. b. ____________________ -neutral particle. c. ____________________ -negatively charged particle. d. The ____________________ is almost two thousand times larger than the ____________________. e. The ____________________ has the same mass as the ____________________. Atomic models are used to show what the atom looks like because an atome is too small to see. In this class we use Bohr’s model of the atom because it represents the behavior of the atom and is fairly easy to understand. Remember that the Bohr model shows the nucleus surrounded by electrons in their electron orbitals. Below is a diagram of a Helium atom using Bohr’s model. 2). Complete the following statements by unscrambling the letters in the blanks. a. The center part of the atom is the UECLUNS ____________________. b. Although the Bohr Model shows electrons as being in orbitals around the nucleus, they are actually in an TECLENOR DOULC ____________________ ____________________ that surrounds the nucleus. c. Protons and RENOUTNS ____________________ are found in the nucleus. d. The nucleus is positively charged because it contains SNOROPT. ____________________ e. There is a force of attraction between the TOVIIPE ____________________ nucleus and the VATGIEEN ____________________ electron orbitals. 3). Complete the sentences by filling in the blanks with the correct words. a. The positive charge of a nucleus is +7. The negative charge that can neutralize it is ____________________. b. In an atom, the number of protons in the nucleus is called the ____________________. c. The number of electrons in the electron orbitals is the same as the number of ____________________. d. The atom is electrically ____________________, even though it contains positive and negative charges. e. Each element can be identified by its ____________________.