* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter2. Elements of quantum mechanics
Hidden variable theory wikipedia , lookup
Renormalization wikipedia , lookup
Canonical quantization wikipedia , lookup
Tight binding wikipedia , lookup
Bremsstrahlung wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Particle in a box wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
Atomic orbital wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Hydrogen atom wikipedia , lookup
Electron configuration wikipedia , lookup
Double-slit experiment wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Atomic theory wikipedia , lookup
Matter wave wikipedia , lookup
Wave–particle duality wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
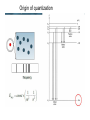
Chapter2. Elements of quantum mechanics Present outline Classical mechanics 1. An object in motion tends to stay in motion. 2. Force = mass times acceleration 3. For every action there is an equal and opposite reaction. Newton Classical mechanics is “everyday life” mechanics. Classical macroscopic particles Propagating plane wave Huygens’ principle Propagating plane wave : Light is an Electromagnetic wave Standing wave Frequency content of light Quantum mechanics: when? 1 meter Classical mechanics 1 millimeter Classical mechanics 1 micrometer Classical mechanics 1 nanometer Quantum mechanics Black body radiation A solid object will glow or give off light if it is heated to a sufficiently high temperature. Figure 2.1 Wavelength dependence of the radiation emitted by a blackbody heated to 300K, 1000K, and 2000K. Note that the visible portion of the spectrum is confined to wave lengths 0.4㎛ ≤λ≤0.7㎛. The dashed line is the predicted dependence for T=2000K based on classical considerations. Origin of quantization The Bohr atom Postulation by Bohr 1. Electrons exist in certain stable, circular orbits about the nucleus. 2. The electron may shift to an orbit of higher or lower energy, thereby gaining or losing energy equal to the difference in the energy levels. 3. The angular momentum Pθ of the electron in an orbit is always an integral multiple of Planck’s constant divided by 2π 18/39 – The energy difference between orbits n1 and n2 Figure 2.2 Hydrogen energy levels as predicted by the Bohr theory and the transitions corresponding to prominent, experimentally observed, spectral lines. – Atomic Spectra • The analysis of absorption and emission of light by atoms Paschen Balmer Lyman λ (thousands of angstroms) A series of sharp lines rather than a continuous distribution of wavelengths • Photon energy h v is related to wavelength by • E=h v = hc ∵c = vλ Photoelectric effect An observation by Plank : radiation from a heated sample is emitted in discrete units of energy, called quanta ; the energy units were described by h, where is the frequency of the radiation, and h is a quantity called Plank’s constant En=nhν=nћω, h = 6.63 × 10-34 J·s, ћ=h/2π Quantization of light by Einstein → photoelectric effect Em : a maximum energy for the emitted electrons Em = hν - qΦ ( Φ : workfunction ) Workfunction : the minimum energy required for an electron to escape from the metal into a vacuum Photoelectric effect A photoelectric experiment indicates that violet light of wavelength 420 nm is the longest wavelength radiation that can cause photoemission of electrons from a particular multialkali photocathode surface. a. What is the work function of the photocathode surface, in eV? b. If a UV radiation of wavelength 300 nm is incident upon the photocathode surface, what will be the maximum kinetic energy of the photoemitted electrons, in eV? c. Given that the UV light of wavelength 300 nm has an intensity of 20 mW/cm2, if the emitted electrons are collected by applying a positive bias to the opposite electrode, what will be the photoelectric current density in mA cm-2 ? Solution a. We are given max = 420 nm. The work function is then: = ho = hc/max = (6.626 10-34 J s)(3.0 108 m s-1)/(420 10-9 m) = 4.733 10-19 J or 2.96 eV b. Given = 300 nm, the photon energy is then: Eph = h = hc/ = (6.626 10-34 J s)(3.0 108 m s-1)/(300 10-9 m) Eph = 6.626 10-19 J = 4.14 eV The kinetic energy KE of the emitted electron can then be found: KE = - Eph = 4.14 eV - 2.96 eV = 1.18 eV c. The photon flux ph is the number of photons arriving per unit time per unit area. If Ilight is the light intensity (light energy flowing through unit area per unit time) then, ph =Ilight/Eph Suppose that each photon creates a single electron, then J = Charge flowing per unit area per unit time = Charge Photon Flux = 48.4 A m-2 = 4.84 mA cm-2 Electron impact excitation a. A projectile electron of kinetic energy 12.2 eV collides with a hydrogen atom in a gas discharge tube. Find the n-th energy level to which the electron in the hydrogen atom gets excited. b. Calculate the possible wavelengths of radiation (in nm) that will be emitted from the excited H atom in part (a) as the electron returns to its ground state. Which one of these wavelengths will be in the visible spectrum? Wave - particle duality De Broglie’s Insight de Broglie postulated the existence of matter wa ves. He suggested that since waves exhibit particle-like behavior, then particles should be expected to show wave-like properties. de Broglie suggested that the wavelength of a particle is expressed as = h /p, where p is the momentum of a particle Wave-particle Duality – Compton effect E=hν=mc2, P=mc=hν/c=h/λ • The change in frequency and the angle corresponds exactly to the results of a “billiard ball” collision between photon and an electron – De Broglie : matter waves → wave-particle duality principle Figure 2.3 Constructive interference of waves scattered by the periodic atoms. • We will use wave theory to describe the behavior of electrons in a crystal. Scanning electron microscope