* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Walter_4309 r1.qxd:Layout 1 - Structural Heart Research
Survey
Document related concepts
Transcript
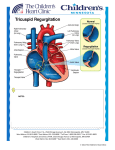
Walter_4309 r1.qxd:Layout 1 21/3/11 14:54 Page 184 Creation of a Tricuspid Valve Regurgitation Model from Tricuspid Annular Dilatation using the Cardioport Video-Assisted Imaging System Eva Maria Delmo Walter1,2, Nikolay V. Vasilyev1, Bjoern Sill1, Muralidhar Padala3, Jorge Jimenez3, Ajit P. Yoganathan3, Roland Hetzer2, Pedro J. del Nido1 1 2 Department of Cardiac Surgery, Children’s Hospital Boston/Harvard Medical School, Boston, Massachusetts, USA, Deutsches Herzzentrum Berlin, Berlin, Germany, 3Georgia Institute of Technology, Atlanta, Georgia, USA Background and aim of the study: Experimental models of tricuspid valve regurgitation (TR) are used to study novel annuloplasty techniques (including prosthetic rings), and they can also serve as physiologic models to investigate TR pathophysiology. The study aim was to develop an appropriate simple and reproducible experimental model of TR from annular dilatation. Methods: Acute TR was successfully created through multiple small 3- to 5-mm incisions in the annulus using a custom-made optical port with an instrument shaft (the Cardioport) that accepts a standard endoscopic imaging system. The Cardioport was inserted, via a thoracotomy, through the right atrium of seven Yorkshire pigs, and directed towards the tricuspid valve annulus to create the annular incisions. Tricuspid valve anatomy and function were evaluated using 2D and 3D echocardiography. The presence and severity of TR, annulus diameter, and changes in heart rate and atrial pressures after making the annu- lar incisions were documented. To monitor tricuspid annular dilatation and the progression of TR, follow up echocardiography and color Doppler examinations were performed at two and eight weeks postoperatively. Results: The acute onset of TR was well tolerated, and there were no deaths or significant morbidity associated with the procedure. The annular diameter was increased from a preoperative mean of 23.1 ± 1.7 mm, to 32.2 ± 2.5 mm at two weeks postoperatively, and to 37.3 ± 3.6 mm at eight weeks postoperatively. Overall, the TR progressed from mild (grade I) to severe (grade III) in all of the animals. Conclusion: This novel porcine model represents a relatively simple and a reproducible surgical technique for the creation of annular dilatation and TR, and may also serve as a chronic model of the latter condition. In the past, relatively few methods have been described for the creation of subacute valvular insufficiency in animal models. Those presented usually have limitations, such as permanent destruction of the valve leaflets or chordae, or a lack of control over the degree of regurgitation produced. Previously, the creation of acute tricuspid regurgitation (TR) has been achieved mainly by using surgical techniques, with the objective of studying the influence of TR on heart hemodynamics or the function of other organs (1-4). Likewise, a percutaneous approach to the creation of TR has been described in a canine model by avulsing the papillary muscles using a biotome (5), and in a similar ovine model by using a catheter (6). However, these were acute studies, as the sudden onset of severe TR was not well tolerated and hence the use of such models to evaluate chronic TR was not pursued. For an evaluation of the effects of tricuspid annular ring implantation to correct regurgitation, a progressive but stable chronic animal model is required. hence, the study aim was to develop a simple and reproducible experimental model of progressive TR from annular dilatation. Presented at the 39th Annual Meeting of the German Society of Cardiothoracic and Vascular Surgery. 18th February 2010, Stuttgart, Germany Address for correspondence: Eva Maria B. Delmo Walter, Children’s Hospital Boston, Harvard Medical School, 300 Longwood Avenue, Boston, Massachusetts 02115, USA e-mail: [email protected] The Journal of Heart Valve Disease 2011;20:184-188 Materials and methods Animals Seven Yorkshire pigs (average body weight 40 kg) were sedated with intravenous ketamine hydrochloride (0.9 mg/kg; Fort Dodge Animal Health, Fort Dodge, © Copyright by ICR Publishers 2011 Walter_4309 r1.qxd:Layout 1 21/3/11 14:54 Page 185 J Heart Valve Dis Vol. 20. No. 2 March 2011 A B C Figure 1: A) The Cardioport with the imaging system. B) Creation of the incision on the tricuspid valve annulus, as viewed from the right atrium using the Cardioport imaging system. C) Tricuspid valve, showing the areas where multiple small incisions and additional incisions were placed. In this way, important landmarks such as the bundle of His and atrioventricular (AV) node are avoided. Iowa, USA) and midazolam hydrochloride (0.05 mg/kg; Bedford Laboratories, Bedford, Ohio, USA). Atropine sulfate (0.02 mg/kg; American Regent Laboratories, Shirley, NY, USA) was also administered intravenously. The animals were administered 5% isoflurane (Isothesia; Burns Veterinary Supply, Rockville Center, NY, USA) via a mask until they were Experimental tricuspid regurgitation L. Walter et al. 185 intubated. Following intubation, the anesthesia was maintained with 2-2.5% isoflurane mixed with oxygen. Arterial pressure lines and venous pressure lines for monitoring were placed percutaneously in the femoral artery and veins, respectively. Enrofloxacin (2.5 mg/kg; Bayer Health Care LLC, Shawnee Mission, KS, USA) was administered intravenously after induction of anesthesia, and a further dose was given postoperatively. The study protocol was approved by the Children’s Hospital Boston/Harvard Medical School Animal Care and Use Committee. Surgical technique to create tricuspid regurgitation Under general anesthesia, the animal was placed in the right anterolateral thoracotomy position, the chest and back were shaved and prepared with povidoneiodine, and sterile drapes were placed in position. An anterolateral thoracotomy incision was made at the third intercostal space, after which the pericardium was incised, sparing the phrenic nerve, to expose the heart. The heart structures (superior and inferior vena cava and right atrium and appendage) were identified, and a purse-string suture (Prolene 4-0) was placed around the atrial appendage. The Cardioport (Fig. 1A), which was developed in the experimental laboratory of the Children’s Hospital Boston/Harvard Medical School, and used previously for intracardiac beating-heart procedures (7), was fitted with a standard video-endoscopic equipment imaging system (Smith and Nephew, Dyonics, Inc., Andover, MA, USA) and a 5-mm rigid 30° telescope (Karl Storz GmbH & Co. KG, Tuttlingen, Germany). The unit was introduced into the right atrium and secured with a Rommel tourniquet. Intraoperative epicardial echocardiography using the X7-2 matrix transducer on an IE33 system (Philips Healthcare, Andover, MA, USA) was carried out to determine the right atrial and right ventricular sizes, the function of the tricuspid valve, and the annular dimensions. Under echocardiographic guidance and direct cardioscopic imaging, multiple (five to ten) small incisions (each of 3-5 mm) were placed circumferentially along the tricuspid annulus, avoiding delicate structures such as the atrioventricular node, the tendon of Todaro, and the Bundle of His (Fig. 1B and C). Intraoperative echocardiography was performed to determine if TR had been induced and, if not, then a further three to five small incisions were added until mild to moderate TR was observed when using color Doppler interrogation. The presence and severity of regurgitation, the diameter of the annulus, and changes in the heart rate and right atrial pressures after annular incisions, were each documented. After confirming that no damage had been caused to the valve leaflets or subvalvular apparatus, the cardioscope was Walter_4309 r1.qxd:Layout 1 21/3/11 14:54 Page 186 186 Experimental tricuspid regurgitation J Heart Valve Dis Vol. 20. No. 2 March 2011 L. Walter et al. fentanyl patch (Ortho-McNeil-Janssen Pharmaceutical, NJ, USA) was applied to the back of each animal to provide postoperative analgesia. When awake and recovered, the pig was returned to the animal house in a stable hemodynamic condition, with all pressure lines and chest tubes having been removed. Cefuroxime (500 mg twice daily) was administered orally for three days to each pig as antibiotic cover. Two-dimensional (2D) and three-dimensional (3D) echocardiography with color flow Doppler were performed at two and eight weeks postoperatively to monitor the progression of annular dilatation and the severity of the TR. A Statistical analysis All data were analyzed using SPSS 16.0 (SPSS Inc., Chicago IL, USA) software. The data were expressed as absolute frequency values, and continuous data as mean ± SD, as appropriate. Time-related events were examined using the Friedman test at 95% confidence intervals (CI). B Results Figure 2: A) Preoperative echocardiography showing a normal right atrial and right ventricular anatomy, with normal valve leaflet morphology, mobility and without prolapse, and normal annular diameter. B) Post-incision onset of acute tricuspid regurgitation withdrawn from the right atrium, and the purse-string suture with additional sutures if necessary. Chest tubes were placed along the pleural space, and the posterior pericardium and pericardium were closed. The ribs were approximated and chest was closed in layers. A Preoperative echocardiographic studies showed normal right atrial and right ventricular anatomies, with normal valve leaflet morphology, mobility and without prolapse in all animals (Fig. 2A). There was no tricuspid regurgitation, and the mean annular size was 23.1 ± 1.7 mm (range: 21 to 25 mm). The successful creation of acute TR was documented and quantified with continuous-wave and color Doppler echocardiography (8) (see Table I and Fig. 2B). The TR was well tolerated by all animals, and no morbidity or mortality was recorded. Echocardiographic studies conducted at two and eight weeks postoperatively demonstrated a progressive increase in the annular dimensions (Figs. 3A and 4A). Postoperatively, the mean annular dimension was 32.2 ± 2.5 mm (range: 27 to 36 mm) after two weeks, and 37.3 ± 3.6 mm (range: 36 to 43 mm) after eight weeks. The TR also increased progressively in severity (Figs. 3B and 4B), from a mean grade of 1.6 ± 0.5 to grade 3 in all animals, at two and eight weeks, respectively. Table I: Severity of tricuspid regurgitation (TR): Echocardiographic data and Doppler parameters. Parameters/location Jet area-central jet (cm2) Jet density and jet continuous wave Severity of TR Mild Moderate Severe <5 5-10 >10 Soft and parabolic Dense, variable Transparent, triangular (with early peak) Walter_4309 r1.qxd:Layout 1 21/3/11 14:54 Page 187 Experimental tricuspid regurgitation L. Walter et al. J Heart Valve Dis Vol. 20. No. 2 March 2011 A 187 A B B Figure 3: Postoperative increases in (A) the annular dimensions and (B) the severity of tricuspid regurgitation. Figure 4: A) Development of annular dilatation over time. B) Development of TR severity over time. Discussion The animal model developed to create TR by inducing annular dilatation with multiple small annular incisions is relatively simple, does not require cardiopulmonary bypass, avoids injury to the valve leaflets or subvalvular apparatus and, in the present authors’ experience, was not associated with complications. The model was easily reproducible, and resulted in a predictable progression of TR. Notably, the technique does not involve technical complexities such as laceration of the valve leaflets, transection or avulsion of the papillary muscles or chordae tendineae, nor suture ligation of the valve leaflets (3,4,6). Such previously described techniques all have the limitation of causing permanent damage to the critical valve structures, and they also lack control over the degree of TR produced. The concept of annular incisions has been described previously, though only in an ex vivo model with the mongrel dog heart to develop modifications of the DeVega annuloplasty technique (1). The present model was created in the beating heart, and the results obtained describe the progression of TR with this technique in a porcine model. The multiple surgical incisions, as made in the present study, can be placed with accuracy around the annulus, and controlled precisely by employing a combination of ultrasound imaging and the Cardioport optical imaging system. The multiple annular incisions Walter_4309 r1.qxd:Layout 1 21/3/11 14:54 Page 188 188 Experimental tricuspid regurgitation L. Walter et al. eventually induced progressive annular dilatation over time, which led consequently to an increasing severity of the TR. Moreover, the process was easily monitored by using serial transthoracic echocardiography. Importantly, echocardiographic imaging at follow up showed that no injuries had occurred to either the valve leaflets or the subvalvular apparatus. The present animal model could be used for hemodynamic studies of acute TR as well as for the evaluation of chronic TR, and the effect of surgical or catheter-based interventions. This novel in vivo porcine model may be used to facilitate research on the development of annuloplasty rings to treat annular dilatation, or potentially to create novel procedures for the treatment of tricuspid valve dysfunction. In conclusion, this novel in vivo porcine model is safe, relatively simple, easily reproducible, and provides predictable results, allowing the creation of TR from annular dilatation, without significant associated morbidity or mortality. Acknowledgments Funding for the development of the Cardioport was provided by grants from the Center for Integration of Medicine and Innovative Technology (CIMIT) and from the Massachusetts Technology Transfer Center (MTTC). The studies were supported financially by the Coultier Foundation Translational Research Award. The authors are grateful to the ARCH staff (Arthur Nedder, Mark Kelly, Kathryn Mullen, Kimberlie Hauser) of the Children’s Hospital Boston/Harvard Medical School for their overwhelming support and assistance in the project. They also thank Herr Helge Haselbach, of the Department of Graphics and Photography of the Deutsches Herzzentrum, Berlin, Germany. J Heart Valve Dis Vol. 20. No. 2 March 2011 References 1. Otaki M, Lust RM. Modification of DeVega’s tricuspid annuloplasty for experimental tricuspid regurgitation. J Card Surg 1994;9:399-404 2. Buffington CW, Nystrom EU. Neither the accuracy nor the precision of thermal dilution cardiac output measurements is altered by acute tricuspid regurgitation in pigs. Anesth Analg 2004;98:884-890 3. Kinney TE, Olinger GN, Sagar KB, Boerboom LE. Acute, reversible tricuspid insufficiency: Creation in a canine model. Am J Physiol 1991;260: H638-H641 4. Yamada T, Hirose H, Umeda S, Murakawa S, Mori Y, Hashimoto M. Modified technique for chronic tricuspid regurgitation in rabbits and evaluation of intestinal malabsorption in the chronic stage of this model. Nippon Kyobu Geka Gakkai Zasshi 1992;40:896-900 5. Baumann RP, Rembert JC, Greenfield JC, Jr. Myocardial blood flow in awake dogs with chronic tricuspid regurgitation. Basic Res Cardiol 1998;93:63-69 6. Hoppe H, Pavcnik D, Chuter T, et al. Percutaneous technique for creation of tricuspid regurgitation in an ovine model. J Vasc Intervent Radiol 2007;18:133-136 7. Vasilyev NV, Martinez JF, Freudenthal FP, Suematsu Y, Marx GR, del Nido PJ. Three-dimensional echo and videocardioscopy-guided atrial septal defect closure. Ann Thorac Surg 2006;82:1322-1326 8. Ionac A, Popescu I. Echocardiographic assessment of ‘forgotten valve’. Timisoara Med J 2007;57:81-87