* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Nuclear_Chem_016
Nuclear fission product wikipedia , lookup
Background radiation wikipedia , lookup
Fallout shelter wikipedia , lookup
Nuclear fission wikipedia , lookup
Ionizing radiation wikipedia , lookup
Technetium-99m wikipedia , lookup
Nuclear and radiation accidents and incidents wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Valley of stability wikipedia , lookup
Nuclear drip line wikipedia , lookup
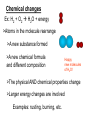
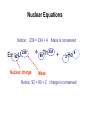
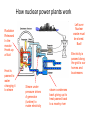
Nuclear Chemistry Throughout history people have tried to control the chemical matter in their world. In order to do so, an understanding of the nature of matter; its inner-workings is of course necessary. Nuclear Chemistry The ultimate goal of changing lead to gold, as was the alchemists’ ambition was never reached. An understanding of the world of the nucleus is necessary, and we are still engaged in that endeavor to this day. Changes: physical To understand the nucleus and its changes it is necessary to review physical and chemical changes. In physical changes such as melting or boiling, the structure of the molecules themselves does not the change. As a result the chemical formula remains the same, and in general only gross physical properties change. Scientist agree: Climate change happens! Polar bears depend on ocean ice to hunt seals. Polar bears may be extinct within our lifetime H2O(s) H2O(l) Changes: physical Illustrate the melting of ice with a particle diagram: Scientists agree; climate change happens! H2O(s) H2O(l) Physical Changes Ex: H2O (s) + energy H2O (l) >Molecules arrangement with each other changes >The physical properties change >The chemical formula and the composition remains unchanged The crystal structure of ice breaks down, but its still H2O! >Small energy changes occur Examples: Melting, dissolving ,etc. Changes: chemical In chemical changes the molecular structure itself is changed as the atoms rearrange into new combinations. As a result new molecules are formed, with a completely different chemical formula and a host of new properties, depending on the new arrangement of atoms. H 2 + O 2 H 2O Changes: chemical This type of change may or may not involve a transfer of electrons between the atoms involved, leading to a more stable arrangement of electrons. Do you recall that atoms lose or gain electrons typically to become like noble gases? H 2 + O 2 H 2O Changes: chemical Illustrate the reaction of hydrogen with oxygen to form water using a particle diagram: H’s O’s H 2 + O 2 H 2O H 2 + O2 H 2O Be sure to apply the law of conservation of mass Chemical changes Ex: H2 + O2 H2O + energy >Atoms in the molecule rearrange >A new substance formed >A new chemical formula and different composition Happy new molecules of H2O! >The physical AND chemical properties change >Larger energy changes are involved Examples: rusting, burning, etc. So it is that in neither physical nor chemical changes, has the nucleus’ arrangement of neutrons and protons changed. The nucleus in a sense is like the world of Dr. Seuss and the whos. It’s a place unaffected by outside factors like temperature and pressure. The nucleus is as unaware of the changes that occur in the chemical world outside, as we are of the changes that occur within. It is a world governed by unimaginably strong forces, the likes of which we’ve only just begun to understand. Welcome to the strange world of nuclear chemistry 1 - Nuclear Changes > Nuclear particles rearrange Watch carefully below Can you spot a neutron changing into a proton? In the example, an unstable carbon 14 nucleus changes into a stable Nitrogen 14 nucleus. Nuclear Changes > Nuclear particles rearrange As nuclear particles rearrange radiation particles are given off. In this case a beta particle is released. Nuclear Changes > Nuclear particles rearrange Changes such as this nuclear decay, involve nuclear particles changing forms: In this case neutrons are changing into protons. Nuclear Changes > Nuclear particles rearrange > Atomic number can change and Old elements can change into new elements When the number of protons change the atomic number and identity of the element change Nuclear Changes > Nuclear particles rearrange > Atomic number can change and Old elements can change into new elements >Mass is converted into energy (as Radiation) The radiation that is given off comes when matter is converted into energy. The famous E=mc2 equation of Einstein shows the relationship Nuclear Changes > Nuclear particles rearrange > Atomic number can change and Old elements can change into new elements > Mass is converted into energy (as Radiation) > Largest energy changes Nuclear reactions like this fusion explosion release much more energy than even the most violent of chemical changes. Nuclear Changes > Nuclear particles rearrange > Atomic number can change and Old elements can change into new elements >Mass is converted into energy (as Radiation) > Largest energy changes A more useful nuclear change is fission, which can provide huge amounts of electricity and doesn’t contribute to climate change. Nuclear Changes > Nuclear particles rearrange > Atomic number can change and Old elements can change into new elements > Mass is converted into energy (as Radiation) > Largest energy changes Examples: radioactive decay, fission, fusion It has other undesirable consequences though. Nuclear Stability > Stability of the nucleus depends on ratio between protons and neutrons > Stable ratio is fairly equal > (1proton :1neutron) in smaller atoms, > About 1 : 1.3 ratio in larger atoms Ex: Bromine 80 = 35 protons : 45 neutrons Nuclear Stability Ex: Carbon 12 6 protons 6 neutrons Stable nucleus Ex: Carbon 14 6 protons 8 neutrons Unstable nucleus Think of the nucleus like a blob of cookie dough. Not enough water and dough crumbles, too much water and its too runny to stay together. The nucleus requires a particular ratio of protons to neutrons to be a stable unit. The graphic below shows the numbers of neutrons and protons for specific nuclides or C, N, Ne, and S Explain, in terms of atomic particles why the S-32 shown in the graph is stable Radioactivity: 1) Transmutation or “decay” Many different kinds of radiation Particles can be produced Transmutation also known as “decay” occurs when particles in an unstable nucleus break down releasing a particle of radiation. Radioactivity: Transmutation or “decay” Many different kinds of radiation Particles can be produced > All atoms above atomic number 84 have an unstable nucleus. In the illustration an unstable Uranium nucleus decays, releasing a particle of radiation, Radioactivity: Transmutation or “decay” Many different kinds of radiation Particles can be produced Though Uranium releases neutrons, a variety particles of radiation are released by other elements. These include alpha particles, protons, and negative beta particles (like electrons from the nucleus!). Pure energy can also be released in the form of gamma radiation. Particles: Reference table O: Alpha particles – (alpha decay) > Largest particle, with mass of 4 and +2 charge >Similar to helium nucleus: 2 He 4 >Greek symbol alpha α upper: Mass # Lower: Atomic # (“nuclear charge”) Ex: Alpha emitter: Pu-234 240 Pu 94 236 + He 4 U 92 2 Alpha particle This is a nuclear equation. In nuclear equations, the atoms change, so an element present on one side, will be a different element on the other side. Ex: Alpha emitter: Pu-234 240 Pu 94 236 + He 4 U 92 2 Alpha particle Notice though,how the atomic numbers (charge #) and mass numbers balance. More on this later! Notice: unstable Plutonium loses 2 protons, becomes uranium Beta particles (Beta Decay) >Small particles with little mass and -1 charge >Similar to electron >Greek symbol β 0 e -1 Notice the lower Number is the charge Beta Emitter: Carbon 14 14 N 14 + C 6 7 0 e -1 Beta radiation Notice: Carbon loses a negative charge and ends up with an extra proton (Atomic number 6 changes to 7) How? A neutron loses a negative charge and becomes a proton! Gamma Radiation >Highest energy particle, similar to x -rays >Has no apparent mass or charge (neutral) >Symbol gamma: γ No loss of charge or mass means element doesn’t change (Though its usually given off with other particles) Ex: Cobalt-60 emits gamma rays in addition to beta electrons Selected Radioisotopes Shown in Table N (radioactive, unstable) Radiation forms Try this problem: (we’ll do more of these. Later) What particle is represented by X? 11 11 C B +X Solution: Insert atomic numbers (from per. Table) and missing mass numbers to balance 0 C B + X 6 5 +1 11 11 0 mass and +1 charge? In nuclear equations numbers must balance on both sides. +6 nuclear charge on the left. We insert a +1 on the right to balance. The mass of 11 on the left balances with the 11 on the right. The particle X must have a mass of zero. Try this problem: (we’ll do more of these. Later) What particle is represented by X? 11 11 C B +X Solution: Insert atomic numbers (from per. Table) and missing mass numbers to balance 0 C B + X 6 5 +1 11 11 According to table O It’s a positron Anti-matter! Produced in particle accelerator 0 mass and +1 charge? Try one: But don’t peek! Notice: 1) U-238’s mass decreases by 4, nuclear charge decreases by 2: Loss of alpha particle +2He4 2) Th234’s mass stays same, 0 mass lost, but nuclear charge increases by +1: loss of -1 charge: -1e0 beta particles. 3) Same change for Pa-234 turning into U-234 Penetration of particles >Alpha’s are the largest (mass of 4), so they penetrate little >Beta’s are smaller, (mass of 0, like an electron) penetrate easier >Gamma’s smallest (pure energy), highest penetration Keep and eye peeled for this paper, wood, and concrete example What stops the radiation? Indian point nuclear power plant in Peekskill What are the silos made from? Why? Separation of particles >Alpha’s are positive, deflected toward negative plate >Beta’s are negative, deflect toward positive side >Gamma’s are neutral, undeflected. This website shows animates the separation of particles Can you tell which particle is which? Test your learning 1) How are physical changes different from chemical changes? How does the nucleus change during those changes? 2) What are the main characteristics of nuclear changes? 3) What characteristic of an atom’s nucleus causes it to be unstable? 4) What are the three naturally produced radiation forms? What are their properties? Click here to check out the crash course take on this topic The energy released by a nuclear reaction results primarily from the (1) breaking of bonds between atoms (2) formation of bonds between atoms (3) conversion of mass into energy (4) conversion of energy into mass The stability of an isotope is based on its (1) number of neutrons, only (2) number of protons, only (3) ratio of neutrons to protons (4) ratio of electrons to protons Which of these types of radiation has the greatest penetrating power? (1) alpha (3) gamma (2) beta (4) positron 2- Half-life >Radioisotopes decay at a special rate >Used to age geologic and fossil samples Example: (Ref Table H) Radium 226 Half-Life = 1600 years After Time # half-lives % sample remaining Fraction remaining Mass remaining 0 0 100 all ½ left 10.00 g After 2 half-life 25% of original ¼ left 2.50 g left After 3 half-life 12.5% of original 1/8 left 1.25 g left after1600 y After 1 half-life 50% of original after 3200 y +1600= after 4800 y +1600= 5.00 g left Suppose that we start with 10 grams of Ra-222 Half-life time is listed on reference table N Carbon-14’s ½ life is 5,700 years Uranium-238’s ½ life is 4.5 billion years Which would work better to find the age of ancient rocks? (hint: the earth is billions of years old!) Answer: U-238. half–life closer to target age. Graphing radioactive decay Starting mass. After one Half-life. What fraction is left after the second half-life? Question: According to the graph, What is the half-life of Cesium 137? Original sample is 100 grams, drops to 50 grams after 30 years. Fraction remaining problems Ex: What fraction of a sample of K-42 remains unchanged after 49.6 hours? Ref. Table N: Half life K-42 = 12.4 hrs Total time Half-life time 49.6 hrs = 4 half-lives 12.4 hrs Fraction Remaining = (1/2) 4 (Number of half-lives) = (1/2) =½x½x½x½ = 1/16th Or make a table: time 0 7.2 sec 14.4 sec 21.6 sec 28.8 sec 36.0 sec fraction remaining = (1/2)5 (1/2)36/7.2 =½x½x½x½x½ fraction all 1/2 1/4 1/8 1/16 1/32 Here’s another: Based on the selected radioisotopes chemistry reference table, what is the fraction of a sample of potasium-42 that will remain unchanged after 62.0 hours? time fraction 0 12.4 hrs all 24.8 hrs 1/4 37.2 hrs 1/8 49.6 hrs 1/16 62.0 hrs 1/2 1/32 Nuclear Equations Notice: 238 = 234 + 4 Ex: 92 U 238 Nuclear charge Mass is conserved 234 Th + 90 4 He 2 Mass Notice: 92 = 90 + 2 charge is conserved Nuclear equations can be balanced just like chemical equations. The problem below illustrates the process. Problem: What is the identity of X? 226 Ex: 88 Ra 226 88 = ? = X ? + 4 + 4 He 2 + 2 222 X 86 Atomic number 86 = Radon 222 Rn 86 Test your learning 1) The half life of iodine-125 is 60 days. What fraction of iodine-125 nuclides would be left after 360 days? 2) A medical institution requests 1 g of bismuth-214, which has a half life of 20 min. How many grams of bismuth-214 must be prepared if the shipping time is 2 h? 3) Use reference table to write the nuclear equation for the decay of iodine 131. What particle is emitted? What new element is produced? 4) Radon 222 is produced by the decay of what radioisotope? Write a balanced nuclear equation to find out: 0 +1 A positron Regents exam question 79 - 81: 3 - Radioactivity: Artificial Transmutation >Decay caused by bombarding nuclei with outside radiation, in particle accelerators Ex: 13 element Al 27 + 2 He 4 Hit with radiation 15 P 30 + 0 n 1 Forms New element (an Alpha particle) In artificial transmutation, look for a radiation particle on the left, reactant side. Particles to look for include Protons or the like: 1 H 1 and Alpha particles: 2 He 4 And more Radiation (a neutron) Particle accelerators are used study the structure the atom’s nucleus > Particles are accelerated to a high speeds And then collide into various samples. Detectors help analyze the results. This website is the particle adventure. What are subatomic particles made of? The strange world of sub-subatomic particles Aka: What are Protons, neutrons and electrons made of? (not on the regents exam, but who cares? Its weird, cool stuff!) Elementary particles: Quarks (nuclear), leptons (electron) & gluons (force carriers) 6 Quark flavors: up(+2/3 charge) down (-1/3) charm (+2/3) strange (-1/3) top (+2/3) bottom (-1/3) u(+2/3 ) u(+2/3 ) + d(-1/3 ) +1 proton Neutron u(+2/3 ) d(-1/3 ) + d(-1/3 ) 0 FYI: particleadventure.org 2-4 aren’t even nuclear changes, they’re chemical reactions! An easy one, huh? Radioactivity: Nuclear Fission Fission = Splitting the atom’s nucleus Releases large amounts of energy Nuclear Fission > A large nucleus is split into two smaller nuclei More Radiation radiation U fuel 2 products Releases energy In a chain reaction, on neutron strikes a nucleus, splitting it and releasing 3 neutrons. Each of the 3 strikes another nucleus, releasing a total of 9 neutrons. And so on. Imagine the consequences! Chain Reaction 1 3 9 27 etc. Mass is converted into energy during nuclear changes How nuclear power plants work Left over Nuclear waste must be stored. Bad! Radiation Released In the reactor Heats up a fluid Heat is passed to water changing it to steam Electricity is passed along the grid to our homes and businesses Steam under pressure drives A generator (turbine) to make electricity steam condenses back giving up its heat passed back to a nearby river Nuclear Energy Advantages >No gas emissions like with fossil fuels (leading to climate change) >Lots of energy for small amount of fuel Disadvantages >Radioactive waste has long half-lives -Remain dangerous for long periods -Present long term storage problems Nuclear Fusion >Fusion = put together >Two small nuclei combine into one >Release huge amounts of energy 1H 2 3 4 1 + 1 H 2 He + 0 n + energy deuterium and tritium changed into Helium Problem: Positive nuclei repel must use small nuclei used to overcome forces Extremely High temperatures required Nuclear weapons: the H bomb Aka: what good is a device that destroys everything? TNT chemical bomb fission bomb fusion bomb Test your learning 1) Write a nuclear equation showing natural transmutation and an equation showing artificial transmutation. Explain how they are different. 2) Describe one advantage of nuclear fission and one disadvantage. 3) What advantage does nuclear fusion have over nuclear fission? Why don’t we use nuclear fusion for energy production? Can you identify these changes? Fission Natural Transmutation (alpha decay) Artificial Transmutation Fusion synthesis decompostion Artificial Transmutation Natural Transmutation (beta decay) 4 - Uses for Radioisotopes Tracers – track chemical reactions Ex: Photosynthesis with radioactive Oxygen tracer CO2 + H2O + sunlight C6H12O6 + O2 Where does the Oxygen we breath come from? Notice where the O in O2 came from? From the water! (not the CO2) Industrial applications Measuring thickness of material, based on absorption of radiation (thicker materials absorb more radiation) Uses for Radioisotopes Medical Treatments (short half-lives) Technetium 99 – Diagnose brain tumors (tracer) Iodine 131 – Treat thyroid disorders Radium and Cobalt 60 – cancer treatment Food Irradiation kills bacteria and fungi, prevents food spoilage Geologic dating U-238 to Pb-206 ratio – dating rocks C-14 to N-14 ratio – age of fossils Half-life determines its application Test your learning 1) What characteristic of a radioisotope is important for its use in medical procedures? 2) What advantage does irradiated food have for developing countries? 3) What radiation particle is emitted by Technetium 99, allowing it be visible during a bone scan? 4) Why is uranium 238 used to date rock formations while Carbon 14 is used for fossils?