* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download T-Vector Direction Differentiates Postpacing From Ischemic T
Cardiac contractility modulation wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Jatene procedure wikipedia , lookup
Coronary artery disease wikipedia , lookup
Ventricular fibrillation wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
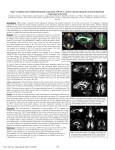
T-Vector Direction Differentiates Postpacing From Ischemic T-Wave Inversion in Precordial Leads Alexei Shvilkin, MD; Kalon K.L. Ho, MD, MSc; Michael R. Rosen, MD; Mark E. Josephson, MD Downloaded from http://circ.ahajournals.org/ by guest on August 13, 2017 Background—Postpacing precordial T-wave inversion (TWI), known as cardiac memory (CM), mimics ischemic precordial TWI, and there are no established ECG criteria that adequately distinguish between the two. On the basis of CM properties (postpacing sinus rhythm T vector approaching the direction of the paced QRS vector), we hypothesized that CM induced by right ventricular pacing would manifest a TWI pattern different from that of precordial ischemic TWI, thereby discriminating between the two. Methods and Results—T-wave axis, polarity, and amplitude on a 12-lead ECG during sinus rhythm were compared between CM and ischemic patients. The CM group incorporated 13 patients who were paced in DDD mode with short atrioventricular delay for 1 week after elective pacemaker implantation. The ischemic group consisted of 47 patients with precordial TWI identified among 228 consecutive patients undergoing percutaneous coronary intervention for an acute coronary syndrome. The combination of (1) positive TaVL, (2) positive or isoelectric TI, and (3) maximal precordial TWI⬎TWIIII was 92% sensitive and 100% specific for CM, discriminating it from ischemic precordial TWI. Conclusions—CM induced by right ventricular pacing results in a distinctive T-vector pattern that allows discrimination from ischemic precordial T-wave inversions regardless of the coronary artery involved. (Circulation. 2005;111:969974.) Key Words: electrocardiography 䡲 pacing 䡲 ischemia T he differential diagnosis of precordial T-wave inversion (TWI) poses a significant clinical challenge. Precordial TWI has a wide range of causes, from a normal ECG variant to hypertrophic cardiomyopathy, pericarditis, and myocardial ischemia.1,2 A syndrome of diffuse precordial TWI associated with critical proximal left anterior descending artery (LAD) disease (sometimes referred to as Wellens’ syndrome3) has been shown to portend a poor prognosis unless the LAD obstruction is definitively treated by percutaneous coronary intervention (PCI) or bypass surgery.2,4 Because the anginal episode often has resolved by the time of presentation,4 physicians confronted with diffuse precordial TWI in an asymptomatic patient often feel motivated to perform urgent catheterization unless a compelling alternative explanation for TWI exists. Cardiac memory (CM) refers to T-wave abnormalities that manifest on resumption of a normal ventricular activation pattern after a period of abnormal ventricular activation, such as ventricular pacing,5 transient left bundle-branch block, ventricular arrhythmias, or WolfParkinson-White syndrome.6,7 Pacing-induced TWI, the most common clinical example of CM, is usually localized to precordial and inferior leads.6,8 Postpacing CM often produces striking precordial TWI that may persist for long periods of time after the pacing is discontinued,5,6 thus obscuring their causal relationship. As a result, TWI due to CM represents an important confounder in the diagnosis of myocardial infarction.6,9,10 No diagnostic features have been described to differentiate pacing-induced CM from TWI that results from ischemia and infarction. Asymptomatic patients with permanent pacemakers who present with pacing-induced precordial TWI often undergo timeconsuming and costly evaluations to rule out ischemia, including unnecessary cardiac catheterization. On the basis of the key attribute of CM (T-wave vector in sinus rhythm approaching the direction of the abnormally activated QRS complex),6 we hypothesized that right ventricular endocardial pacing would result in a precordial TWI with frontal-plane T-vector direction different from that of ischemic precordial TWI. If this were the case, diagnostic criteria for differentiating between the 2 causes could be established. Although precordial TWI was originally described with proximal LAD lesions,2,4 ischemia in the territory of the left circumflex (LCx) and occasionally right coronary artery (RCA) can also cause precordial TWI. Therefore, the purpose of the present study was to establish diagnostic criteria for postpacing versus ischemic precordial TWI independent of the coronary artery involved. Received May 30, 2004; revision received November 17, 2004; accepted November 23, 2004. From the Beth Israel Deaconess Medical Center (A.S., K.K.L.H., M.E.J.), Boston, Mass, and the Center for Molecular Therapeutics (M.R.R.), Columbia University, New York, NY. Correspondence to Mark E. Josephson, Baker4/Cardiology, Beth Israel Deaconess Medical Center, 185 Pilgrim Rd, Boston, MA 02215. E-mail [email protected] © 2005 American Heart Association, Inc. Circulation is available at http://www.circulationaha.org DOI: 10.1161/01.CIR.0000156463.51021.07 969 970 Circulation March 1, 2005 TABLE 1. Distribution of TWI by Infarct-Related Artery in ISC Group Vessel Involved Figure 1. Classification of T waves and calculation of T-wave amplitudes. A through C, Negative T waves (⫺0.8, ⫺0.2, and ⫺0.1 mV, respectively); D, isoelectric T wave (0 mV); E, positive T wave (0.2 mV). LAD TWI, n (%) No TWI Excluded 28 (47)* 31 20 79 Proximal 16 (57) 12 7 䡠䡠䡠 Mid, diagonal 12 (44) 15 10 䡠䡠䡠 0‡ 4 3 12 (21) 44† 17 䡠䡠䡠 73 Distal Methods Total LCx Downloaded from http://circ.ahajournals.org/ by guest on August 13, 2017 Groups of Patients RCA 7 (11) 56 13 76 The CM group consisted of 13 patients followed up prospectively after implantation of a permanent dual-chamber pacemaker who had sinus rhythm with 1:1 AV conduction at physiological heart rates at baseline. CM was induced by 1 week of DDD pacing with a short AV delay to ensure ventricular activation from the endocardial pacemaker electrode positioned in the right ventricular apex. At 1 week, a 12-lead ECG was recorded after the pacemaker was reprogrammed in AAI mode. This ECG was used for analysis. The ischemic TWI (ISC) group of patients was retrospectively identified among the 228 consecutive patients who underwent PCI for a non–ST-elevation myocardial infarction (as identified by symptoms of ischemia and elevated cardiac markers, eg, troponin I, troponin T, or creatine kinase-MB [CK-MB] in the absence of ST elevations on the ECG) at the Beth Israel Deaconess Medical Center from January to November 2003 as noted in the cardiac catheterization laboratory database. Precordial TWI was defined as TWI ⱖ0.1 mV in 2 or more adjacent precordial leads (V1 to V6) on a 12-lead ECG recorded within 48 hours after admission. If TWI was present on ⬎1 ECG, the earliest one was used for analysis. The ISC group was further divided into 3 subgroups (LAD, LCx, and RCA) according to the infarct-related artery. Patients with atrial fibrillation, left bundle-branch block, presence of a pacemaker, multivessel PCI, planned PCI for stable coronary disease, and frequent ventricular ectopy were excluded from the ISC group. Total 47 (26) 131 50 228 ECG Analysis All ECGs were recorded on a Burdick Spacelabs Eclipse Plus ECG (Burdick Spacelabs) at a paper speed of 25 mm/s. The tracings were analyzed manually without magnification. T-wave amplitude was measured by ruler in each lead from T-wave peak/nadir to the baseline determined by T-P segment. In case of biphasic T waves, the most negative deflection was measured, and the T wave was classified as negative. T wave was classified as isoelectric (amplitude⫽0) if both positive and negative components were present with amplitude ⬍0.05 mV (Figure 1). QT was measured manually over 3 consecutive RR intervals in lead II, and the results were averaged. The corrected QT interval (QTc) was calculated according to Bazett’s formula. Frontal-plane QRS and T-vector angles were obtained from the automated standard ECG printouts and not independently determined. All ECGs were analyzed by a single investigator blinded to the patient group allocation. Percentages represent the fraction of nonexcluded tracings with TWI. *P⬍0.05 vs LCx and RCA groups. †Includes 5 patients with isolated TWI in I and aVL. ‡P⬍0.05 vs other LAD locations. pared with 2 test and Fisher’s exact test, as appropriate. These analyses were performed with SPSS 11.5 (SPSS Inc). Angular variables were compared with Watson-Williams F test (Oriana 2.0, KCS Inc). Probability values less than 0.05 were considered statistically significant. Approval for the study was obtained in accordance with the requirements of the Institutional Review Board of the Beth Israel Deaconess Medical Center. All patients undergoing pacemaker implantation signed informed consent for the study. Results ISC Patient Selection A total of 228 patients underwent PCI for a non–ST-elevation myocardial infarction. Fifty patients were excluded (9 with atrial fibrillation, 18 with left bundle-branch block, 12 with ventricular paced rhythm, 3 with multivessel PCI, 7 with planned PCI, and 1 with frequent ventricular ectopy). Forty-seven (26%) of the remaining 178 patients had precordial TWI and made up the ISC group. Precordial TWI was present significantly more often in patients with LAD lesions (47%) than in those with LCx (21%) and RCA lesions (11%; P⬍0.05). TWI was observed in 57% cases of proximal LAD disease and 44% of mid-LAD/ first diagonal branch disease (P⬎0.1) but in no patients with distal LAD lesions (P⬍0.05 versus other LAD locations). (See Table 1.) Baseline ISC and CM group characteristics are presented in Table 2. Age, gender distribution, prevalence of prior MI, TABLE 2. Baseline Patient Data Biochemical Data For LAD ISC patients, the highest level of CK-MB during admission was used for analysis. Group ISC (n⫽47) CM (n⫽13) Male, n (%) 28 (60) 6 (46) Angiographic Data Age, y 65.3⫾2.0 72.5⫾3.0* The culprit coronary artery lesion was determined by a high-volume interventional cardiologist at the time of PCI on the basis of a coronary artery occlusion or stenosis ⬎80% by visual estimation corresponding to the clinical and ECG data. The location of the culprit lesion was determined from angiographic reports and confirmed by visual analysis of digital angiographic images. Prior history of MI, n (%) 12 (26%) 3 (23%) 8 (17) 2 (15) Available 19/47 13/13* Precordial TWI 4/19 0/13 Right bundle-branch block 1/19 3/13 Q waves 4/19 2/13 Statistical Analysis Continuous variables are expressed as mean⫾SEM and compared by ANOVA with Bonferroni correction. Categorical data were com- History of CABG, n (%) Prior ECG *P⬍0.05. Shvilkin et al TABLE 4. T-Wave Inversion 971 Lead Distribution of TWI in ISC and CM Groups ISC Lead Downloaded from http://circ.ahajournals.org/ by guest on August 13, 2017 Figure 2. A, Representative ECG of ISC patient with proximal LAD lesion. This 68-year-old nondiabetic woman, an active smoker with hypertension and strong family history of coronary artery disease, developed progressive angina over the week before admission and was found to have troponin T elevation (1.5 ng/mL, upper limit of normal 0.10 ng/mL) with normal CK-MB level and anterior wall hypokinesis on echocardiogram. Cardiac catheterization revealed 90% proximal LAD stenosis involving origin of first diagonal branch. Her prior ECG was normal. B, Representative ECG of CM patient. This 68-year-old man with obstructive sleep apnea, Wenckebach-type seconddegree AV block, and pauses up to 4 seconds while awake underwent a permanent pacemaker placement 1 week before this recording. Pacemaker was turned off just before recording. Patient has had no history of coronary artery disease and recently had a normal exercise stress test. His preimplantation ECG 1 week earlier showed isolated Q in lead III but otherwise was normal. and CABG did not differ between groups. Nineteen patients in the ISC group (40%) and all patients in the CM group had prior ECGs available. Within that subset of patients, the prevalence of baseline ECG abnormalities (Q waves, right bundle-branch block, TWI) was similar between groups (P⬎0.1). None of the CM patients had symptoms suggestive of ischemia during the study. Within the previous 2 years, 5 CM patients had negative stress test with imaging, and 6 underwent cardiac catheterization that demonstrated the absence of obstructive coronary disease in 3 patients, stable single-vessel occlusion with collaterals in 1, and stable revascularized 3-vessel disease in 2. The remaining 2 patients had both a normal ECG and echocardiogram before pacemaker implantation. In 1 patient, the procedure was compliTABLE 3. LAD (n⫽28) LCx (n⫽12) RCA (n⫽7) CM (n⫽13) V1 8 (29) 1 (8) V2 21 (75) 5 (42) 0* 8 (62) 22 (79) 5 (42) 1 (14)* 12 (92) V4 24 (86) 7 (58) 6 (86) 12 (82) V5 21 (75) 10 (83) 6 (86) 13 (100) V6 16 (57)* 11 (92) 7 (100) 13 (100) I 20 (71)* 11 (91)* 4 (57)* 0 II 8 (29)* 5 (42)* 6 (86) 13 (100) III 3 (11)* 2 (17)* 4 (57)* 13 (100) 1 (8) aVR 10 (36)* 0 1 (14) aVL 23 (82)* 11 (92)* 2 (29) 0 aVF 6 (21)* 4 (33)* 5 (71) 13 (100) (I⫹aVL)† 0* 0* 3 (43)* 13 (100) (I⫹aVL)† and maximal precordial TWI⬎TWIIII 䡠䡠䡠 䡠䡠䡠 0‡ 12 (92) Values are n (%). *P⬍0.05 compared with CM group. †(I⫹aVL) indicates positive T wave in lead aVL, positive or isoelectric T wave in lead I. ‡P⬍0.05 for all ISC patients compared with CM patients. cated by development of pericarditis with moderate pericardial effusion treated conservatively. Representative ECG examples of ISC (LAD lesion) and CM patients are depicted in Figure 2. Both CM and ISC tracings demonstrate deep precordial TWI of similar magnitude and morphology. In addition, the CM patient demonstrates deep inferior TWI. A biphasic T wave in lead II in the ISC tracing is also present. An important difference between recordings appears in leads I and aVL, in which ISC shows TWI, whereas CM manifests positive T waves. ECG data are summarized in Tables 3 and 4. The heart rate and QT and QTc intervals were not statistically different between groups. Among those in the ISC group, LAD lesions produced more pronounced precordial TWI than LCx and RCA lesions both in terms of number of leads affected and maximal amplitude of TWI in a single lead, similar to the findings in CM group (Table 3). Lead distribution of TWI in the CM group was consistent with the T vector following the direction of the paced QRS ISC HR, min ⫺1 LAD (n⫽28) LCx (n⫽12) RCA (n⫽7) CM (n⫽13) 69.4⫾2.1 74.2⫾3.1 66.9⫾3.6 71.7⫾3.7 QT, ms 440⫾10 415⫾11 438⫾10 417⫾10 QTc, ms 415⫾14 377⫾16 418⫾15 371⫾11 No. of precordial leads with TWI Maximal precordial TWI, mV 4.0⫾0.3 ⫺0.45⫾0.06 QRS frontal axis, degrees T-wave frontal axis, degrees 20⫾7 128⫾10* *P⬍0.05 compared with CM group. 5 (39) V3 ECG Data Group 0 3.25⫾0.5* ⫺0.21⫾0.10* 6⫾11 146⫾15* 2.9⫾0.3* ⫺0.26⫾0.11* 6⫾46 ⫺98⫾30 4.8⫾0.3 ⫺0.53⫾0.06 18⫾12 ⫺70⫾5 972 Circulation March 1, 2005 the magnitude of TWI between proximal and mid LAD lesions or between patients with and without D1 territory involvement (data not shown). No patients with distal LAD lesion had precordial TWI. Extent of Myocardial Necrosis CK-MB levels were available in 27of 28 LAD patients. Ten patients had CK-MB within the normal range (⬍10 ng/mL), and 17 (61%) had CK-MB elevation ranging from 13 to 366 ng/mL (median 46 ng/mL, upper limit of normal 10 ng/mL). The magnitude of precordial TWI in patients with normal CK-MB was significantly greater than in those with elevated enzyme levels (P⬍0.01). Discrimination Criteria Between ISC and CM TWI Downloaded from http://circ.ahajournals.org/ by guest on August 13, 2017 Figure 3. Circular histogram of frontal-plane T-axes distribution in LAD, LCx, and CM groups. Solid bars indicate LAD; hatched bars, LCx; open bars, CM. Difference in T-vector axis between CM and LAD/LCx is statistically significant (P⬍0.01). complex. Right ventricular apex pacing produced QRS with left superior frontal-plane axis that was negative in precordial and inferior (II, III, aVF) leads and invariably positive in leads I and aVL. As a result, the postpacing T-wave axis also had a left superior direction (⫺70⫾5 degrees; Table 3). All CM patients had positive TaVL, with diffuse TWI in the precordial and inferior leads. Eleven patients had positive and 2 had isoelectric TI. With the exception of the patient with postimplantation pericarditis, all CM patients demonstrated maximal precordial TWI⬎TWIIII. Lead distribution of TWI in the ISC group was consistent with a T vector directed away from the area of ischemia, with a progressive increase in the frequency of lateral precordial lead involvement from LAD to LCx to RCA culprit vessels (Table 4). The majority of LAD and LCx patients had negative T waves in leads I and aVL. Only 3 LAD/LCx patients had positive T waves in lead I, and 1 patient had a positive T wave in lead aVL, but none had positive T waves in both leads. In vectorcardiographic terms, this translated into a left-to-right frontal-plane direction of the T vector, significantly different from that of CM group (Table 3; Figure 3). Limb-lead TWI pattern in the RCA group was variable, depending on the relative involvement of lateral and inferior leads. Four patients with TWI in leads I or aVL and a left-to-right T-vector direction similar to the LAD and LCx groups had predominantly lateral precordial TWI (maximal precordial TWI ⬎TWIIII). The remaining 3 patients with positive T waves in leads I and aVL had a predominantly inferior-lead TWI (maximal precordial TWI⬍TWIIII). Location of Culprit Lesion in the LAD Group Sixteen patients (57%) with a culprit lesion in the LAD had a proximal LAD lesion with ischemic territory involving the first diagonal (D1) branch. Twelve (44%) had a mid-LAD or isolated D1 lesion. No significant differences were found in CM Versus LAD/LCx All CM patients had positive TaVL compared with only 1 ISC patient (who had a negative T wave in lead I). A positive T wave in lead I was observed in 11 of 13 CM patients; in the remaining 2 (both of whom had prior inferior-wall MI), the T wave was isoelectric. No CM patient had TWI in lead I. The combination of a positive T wave in lead aVL and a positive or isoelectric T wave in lead I was seen in all CM patients and none of the LAD/LCx patients (Table 4). CM Versus RCA Four of 7 RCA patients conformed to the pattern of LAD/ LCx TWI. The remaining 3 RCA patients with positive T wave in leads I and aVL had maximal precordial TWI⬍TWI III, in contrast to all but 1 CM patient. Therefore, the combination of (1) positive TaVL, (2) positive or isoelectric TI, and (3) maximal precordial TWI⬎TWIIII was 92% sensitive and 100% specific for CM, discriminating it from ISC regardless of the coronary artery involved. Discussion We describe an approach to differentiate precordial ischemic TWI from postpacing TWI on the basis of the unique characteristics of the postpacing T-wave vector. The latter is characterized by the left-superior frontal direction, with the magnitude of precordial TWI exceeding that in the inferior leads. We demonstrated that in most cases (LAD, LCx, and the majority of RCA culprit lesions), ischemic precordial TWI is characterized by a rightward frontal-plane T-wave axis. In a small number of patients with inferolateral ischemia with a left T-wave axis, the inferior/precordial TWI ratio still allowed differentiation from CM. Bearing in mind these vector concepts, we devised a simple rule for discriminating CM and ISC precordial TWI using standard 12-lead ECG criteria. The small number of patients in our study (especially those with precordial TWI due to RCA lesions) and the retrospective nature of the analysis make it necessary to confirm these findings in a larger validation cohort. Nevertheless, our data provide the proof of concept for using T vector direction in differential diagnosis of precordial TWI. The ISC group selection criteria served 2 purposes. First, biochemical evidence of myocardial necrosis supported the ischemic nature of the TWI in this group. Second, the presence Shvilkin et al Downloaded from http://circ.ahajournals.org/ by guest on August 13, 2017 of a critical stenosis amenable to PCI minimized the potential ambiguity in culprit lesion identification in cases without total coronary occlusion. At the same time, this could create a bias toward larger ischemic area in the ISC group in the present study, and it is plausible that more localized ischemia could produce different TWI patterns. We found, however, no precordial TWI among 4 patients with distal LAD lesions and presumably smaller areas of ischemia (Table 1). LCx lesions were less likely to produce precordial TWI, and the magnitude and number of leads affected were significantly less than seen with LAD lesions. Nevertheless, LCx ischemia produced a rightward T-wave axis shift similar to that seen with LAD involvement. In fact, among LCx lesions screened for precordial TWI, 5 patients had isolated TWI in I and aVL, demonstrating rightward T-axis direction even without precordial involvement. Therefore, it appears that anterolateral ischemia severe enough to produce precordial TWI results in a rightward frontal-plane T axis. There were very few patients with RCA culprit lesions with precordial TWI in the present study. Although the ratio of inferior/precordial TWI allowed differentiation of inferolateral ischemic TWI and CM, a larger prospective patient sample is required to confirm this observation. Degree of ischemia is another factor potentially affecting the expression of TWI. All ISC patients in the present study had positive biochemical markers of myocardial injury. Conceivably, a milder degree of ischemia could produce smaller T-wave changes. To address this issue, we compared patients with LAD culprit lesions with elevated versus normal CK-MB levels and found the latter to have significantly greater precordial TWI than those with elevated CK-MB. This finding is in accord with observations in patients with myocardial infarction who demonstrate an inverse relationship between TWI magnitude, enzymatic estimate of myocardial infarction size, and functional recovery,11 which suggests that TWI indicates the presence of viable stunned myocardium. Therefore, it appears unlikely that milder ischemia would alter the T-wave pattern in ISC. All CM patients in the present study had endocardial right ventricular apex lead implants. Other positions within the right ventricle can produce different pacing QRS vectors that result in different directions of memory T waves. Right ventricular electrodes can be implanted in the mid septum or right ventricular outflow tract.12,13 The QRS complex produced by pacing from these alternative sites can have variable morphology in leads I and aVL, resulting in a left axis with varying degrees of superior (closer to the right ventricular apex) or inferior (right ventricular outflow tract) angulation. Therefore, postpacing TWI will always assume a left axis, no matter where in the right ventricle the pacing lead is situated. With right ventricular outflow tract pacing, however, one would not see deep TWIs in inferior leads, which are considered typical for postpacing TWI.8 Additional studies are needed to characterize the TWI pattern after pacing from these alternative sites. As demonstrated previously in animal studies, the early stages of CM development can be accompanied by T-vector rotation in the frontal plane before the T wave assumes the T-Wave Inversion 973 direction of the paced QRS complex.14 Therefore, shorter (or intermittent) pacing may result in an “intermediate” T-wave axis. Molecular mechanisms of CM include changes in ion channels such as Ito1,15 IKr,16 L-type Ca channel,17 and the angiotensin II system,18 which result in a change of the transmural repolarization gradient.14 Drugs that affect these systems, such as ACE inhibitors, calcium channel blockers,17 and quinidine,16 influence the development of CM and T-vector shape. It is unclear whether these drugs affect the clinical utility of T-wave patterns in distinguishing ischemic versus nonischemic TWIs. Structural heart abnormalities and their ECG manifestations can alter the expression of CM. In the present study, patients with secondary TWI, such as preexisting left bundlebranch block or left ventricular hypertrophy with repolarization abnormalities, were excluded. Our preliminary observations suggest that CM does not change the abnormal T vector associated with these conditions. CM development might be altered in patients with postinfarction scar. Most molecular and cellular changes responsible for CM development are believed to occur in the proximity of the pacing electrode,19 and therefore a prior inferior-wall myocardial infarction could diminish CM development. Indeed, the only 2 CM patients in the present series with isoelectric rather than positive T waves in lead I had prior inferior-wall infarction. Another example of a structural confounder in the present series was a pericardial effusion blunting the postpacing precordial TWI. At present, we have no data to predict the effect of the combination of ischemia and pacing on T-wave characteristics; therefore, extreme caution should be exercised in interpreting postpacing T-wave changes in patients with symptoms of ischemia. Three CM patients in the present series with known coronary artery disease had angiographically documented stable disease and were asymptomatic; therefore, we felt confident that their T-wave changes were attributed to CM rather than ischemia. Conclusions By applying vectorcardiographic principles to interpretation of a standard 12-lead ECG, we demonstrated that right ventricular apex pacing results in TWI with unique characteristics distinct from those induced by myocardial ischemia. A simple algorithm can be used to differentiate these 2 ECG patterns. The use of such vectorcardiographic information can significantly improve the differential diagnosis of precordial TWIs. Acknowledgment This work was performed during Dr Shvilkin’s Fellowship in Cardiac Pacing and Electrophysiology from the North American Society of Pacing and Electrophysiology. References 1. Okada M, Yotsukura M, Shimada T, et al. Clinical implications of isolated T wave inversion in adults: electrocardiographic differentiation of the underlying causes of this phenomenon. J Am Coll Cardiol. 1994; 24:739 –745. 2. de Zwaan C, Bar FW, Wellens HJ. Characteristic electrocardiographic pattern indicating a critical stenosis high in left anterior descending 974 3. 4. 5. 6. 7. 8. 9. 10. Circulation March 1, 2005 Downloaded from http://circ.ahajournals.org/ by guest on August 13, 2017 coronary artery in patients admitted because of impending myocardial infarction. Am Heart J. 1982;103:730 –736. Rhinehardt J, Brady WJ, Perron AD, et al. Electrocardiographic manifestations of Wellens’ syndrome. Am J Emerg Med. 2002;20:638 – 643. de Zwaan C, Bar FW, Janssen JH, et al. Angiographic and clinical characteristics of patients with unstable angina showing an ECG pattern indicating critical narrowing of the proximal LAD coronary artery. Am Heart J. 1989;117:657– 665. Chatterjee K, Harris A, Davies G, et al. Electrocardiographic changes subsequent to artificial ventricular depolarization. Br Heart J. 1969;31: 770 –779. Rosenbaum MB, Blanco HH, Elizari MV, et al. Electrotonic modulation of the T wave and cardiac memory. Am J Cardiol. 1982;50:213–222. Herweg B, Fisher JD, Ilercil A, et al. Cardiac memory after radiofrequency ablation of accessory pathways: the post-ablation T wave does not forget the pre-excited QRS. J Interv Card Electrophysiol. 1999;3: 263–272. Chou T. Electrocardiography in Clinical Practice. 4th ed. Philadelphia, Pa: WB Saunders; 1996. Gould L, Venkataraman K, Goswami MK, et al. Pacemaker-induced electrocardiographic changes simulating myocardial infarction. Chest. 1973;63:829 – 832. Gould L, Reddy CV, Singh B, et al. T-wave changes with intermittent left bundle branch block. Angiology. 1980;31:66 – 68. 11. Nakajima T, Kagoshima T, Fujimoto S, et al. The deeper the negativity of the T waves recorded, the greater is the effectiveness of reperfusion of the myocardium. Cardiology. 1996;87:91–97. 12. Stambler BS, Ellenbogen K, Zhang X, et al. Right ventricular outflow versus apical pacing in pacemaker patients with congestive heart failure and atrial fibrillation. J Cardiovasc Electrophysiol. 2003;14:1180 –1186. 13. de Cock CC, Giudici MC, Twisk JW. Comparison of the haemodynamic effects of right ventricular outflow-tract pacing with right ventricular apex pacing: a quantitative review. Europace. 2003;5:275–278. 14. Shvilkin A, Danilo P Jr, Wang J, et al. Evolution and resolution of long-term cardiac memory. Circulation. 1998;97:1810 –1817. 15. Yu H, McKinnon D, Dixon JE, et al. Transient outward current, Ito1, is altered in cardiac memory. Circulation. 1999;99:1898 –1905. 16. Plotnikov AN, Shvilkin A, Xiong W, et al. Interactions between antiarrhythmic drugs and cardiac memory. Cardiovasc Res. 2001;50:335–344. 17. Plotnikov AN, Yu H, Geller JC, et al. Role of L-type calcium channels in pacing-induced short-term and long-term cardiac memory in canine heart. Circulation. 2003;107:2844 –2849. 18. Patberg KW, Plotnikov AN, Quamina A, et al. Cardiac memory is associated with decreased levels of the transcriptional factor CREB modulated by angiotensin II and calcium. Circ Res. 2003;93:472– 478. 19. Patel PM, Plotnikov A, Kanagaratnam P, et al. Altering ventricular activation remodels gap junction distribution in canine heart. J Cardiovasc Electrophysiol. 2001;12:570 –577. T-Vector Direction Differentiates Postpacing From Ischemic T-Wave Inversion in Precordial Leads Alexei Shvilkin, Kalon K.L. Ho, Michael R. Rosen and Mark E. Josephson Downloaded from http://circ.ahajournals.org/ by guest on August 13, 2017 Circulation. 2005;111:969-974; originally published online February 14, 2005; doi: 10.1161/01.CIR.0000156463.51021.07 Circulation is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 2005 American Heart Association, Inc. All rights reserved. Print ISSN: 0009-7322. Online ISSN: 1524-4539 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://circ.ahajournals.org/content/111/8/969 Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Circulation can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Circulation is online at: http://circ.ahajournals.org//subscriptions/