* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download file
Marburg virus disease wikipedia , lookup
Hepatitis C wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Neglected tropical diseases wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Tuberculosis wikipedia , lookup
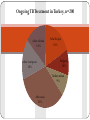
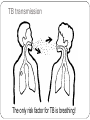
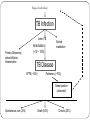
HOW TO PREPARE FOR A PANDEMIC WONCA 2015: THE PRIMARY CARE ROLE Workshop INTRODUCTION Infectious diseases contribute to high morbidity and mortality across the globe due to their nature of spreading through populations and due to varied susceptibility of individual patients. Mass vaccinations, public health measures, general awareness in keeping hygienic measures, modern diagnostics and treatments made a progress in infections disease control and management. However, in a globalised community any outbreak in any region of the world is actually an outbreak of the doorstep of any country or any practice. Recent history tells us how quick a stable situation in infectious disease control can break down. PREPARE PROJECT PREPARE is a EU funded network for harmonized large- scale clinical research studies on infectious diseases (IDs), prepared to rapidly respond to any severe ID outbreak, providing real-time evidence for clinical management of patients and for informing public health responses. PREPARE aims to be at the basis of establishing a paradigm shift in clinical research in response to severe ID outbreaks. AIMS OF THE WORKSHOP To bring some key information on how to approach these challenges from human and biomedical perspective. To discuss primary care role and responsibility in infectious diseases control and possible pandemic. TIMETABLE Introduction: Aims of the course, a brief outline of PREPARE, introducing each other – Zalika Klemenc-Ketis /10 min/ Common infectious diseases in a pandemic: rational decision making in family physicians – Jean-Pierre Jacquet /15 min/ Group work – Experiences with management of infectious diseases and epidemic outbreaks in the participant practices /25 min/ reporting back /5 min/ Traditional and new pathogens in the Middle East immigrants – Mehmet Ungan /15 min/ Summing up /5 min/ Common infectious diseases in a pandemic: rational decision making in family physicians Pr. Jean-Pierre Jacquet Department of General Practice Grenoble University France Pr. Jean-Pierre Jacquet Department of General Practice Grenoble University France No conflict of interest Pr. Jean-Pierre Jacquet Department of General Practice Grenoble University France Seasonal flu Pr. Jean-Pierre Jacquet Department of General Practice Grenoble University France Remember some definitions. Epidemic. Pandemic. Public Health. Prevalence. Incidence Rate. Virulence. Transmission and Communicable disease Pr. Jean-Pierre Jacquet Department of General Practice Grenoble University France What are the potential tasks of the General practitioner/Family? Physician as a researcher? Pr. Jean-Pierre Jacquet Department of General Practice Grenoble University France Four steps: Vaccination Decrease transmission of the virus Identify early cases. Prevent complications. Pr. Jean-Pierre Jacquet Department of General Practice Grenoble University France Vaccination: Rationale Pr. Jean-Pierre Jacquet Department of General Practice Grenoble University France Vaccination, research question: How to increase the percentage of vaccinated people into the risk groups ? What are your proposals? Pr. Jean-Pierre Jacquet Department of General Practice Grenoble University France Vaccination, research question: What are the factors influencing the choice of the patients ? What are your proposals? Pr. Jean-Pierre Jacquet Department of General Practice Grenoble University France Lower transmission of the virus. Rationale Pr. Jean-Pierre Jacquet Department of General Practice Grenoble University France Lower transmission of the virus. Research question: How to change behaviour of the patients to reinforce rules of prevention of inter-human transmission? o What are your proposals? Pr. Jean-Pierre Jacquet Department of General Practice Grenoble University France Identify early cases quickly Rationale Pr. Jean-Pierre Jacquet Department of General Practice Grenoble University France Identify early cases quickly, research question: What are the sensitivity, specificity, positive predictive value and negative predictive value of each symptoms of flu ? What are your proposals? Pr. Jean-Pierre Jacquet Department of General Practice Grenoble University France Identify early cases quickly, research question: How can you set-up a sentinel network ? What are your proposals? Pr. Jean-Pierre Jacquet Department of General Practice Grenoble University France Prevent complications Rationale Pr. Jean-Pierre Jacquet Department of General Practice Grenoble University France Prevent complications, research question: What are the criteria to hospitalise a patient with flu illness? What are your proposals? Pr. Jean-Pierre Jacquet Department of General Practice Grenoble University France Conclusion: A GP can and must do research, even if as non academic it is more difficult. White-Williams-Greenberg diagram, also called the square of White is still relevant, but we have to accept the challenge to produce more scientific data in the field of primary care. Pr. Jean-Pierre Jacquet Department of General Practice Grenoble University France Pr. Jean-Pierre Jacquet Department of General Practice Grenoble University France Bibliography http://www.who.int/influenza/resources/documents/extr act_PIPGuidance09_phase5_6.pdf http://www.who.int/mediacentre/factsheets/fs211/en/ :http://www.who.int/influenza/vaccines/virus/recommen dations/en/ http://www.who.int/ith/diseases/influenza_seasonal/en/ http://www.lecmg.fr/livreblanc/sommaire.html Pr. Jean-Pierre Jacquet Department of General Practice Grenoble University France TOPICS FOR SMALL GROUP WORK Name and describe your experiences with management of infectious diseases and epidemic outbreaks in your country/practice TB CONTROL AND PRIMARY CARE EXAMPLE–WONCA Workshop 2015 Need for Screening? An overview of the region and TB Movement of TB across borders, regional? • Relevant characteristics unique to TB Acknowledgement: Some of the slides are from a CDC tarining presentation in CapeTown by Dr Justin Waring, Medical Director, Western Australian TB Control Program, Chair, National TB Advisory Committee, Australia. Thanks to Dr Waring. WHO 2013 TB REPORT Entry to Turkey-2009 From 2.3 millon to 25.5 million entry to Turkey Entrance to Turkey from Former Soviet Union was 200 000 in 1990 => 5 400 000 in 2009. Balkan countries from 900 000 => 2.6 million Middle East , 400 000 => 2.2 million Entry to Turkey in total was 2.3 million in 1990 => 25.5 million in 2009 Regional Refugee & Resilience Plan UNHCR (3RP) 2015-2016 The projected number of Syrian refugees in Turkey in 2015 is 2.5 million of whom 300,000 will reside in 25 camps and 2.2 million people will live among communities. In addition, it is estimated that 8.2 million people in refugee hosting areas will be impacted. Everyday More and More in Turkey, more than 3 000 000, and impact on at least 8 500 000 in 2015 and 2016 Turkey does a lot….and alone ! U.N.: 850,000 Refugees Are Expected to Reach Europe During 2015 and 2016 Ongoing TB Treatment in Turkey, n=200 other-african 14% other-european 16% Adzerbeijan 18% Bulgaria 10% Turkmenistan 9% other-asia 33% This is only air traffic around the world ! What about the cars, buses, trains and cruises ?? A single country is not able to isolate TB as millions travel in each day and night ! TB transmission The only risk factor for TB is breathing! TB transmission Passive: Individual plays no active part in acquiring the infection Onus for preventing transmission is with health providers Often Unrecognized: Individual does perceive the transmission Individuals often don’t perceive a risk Not the primary reason for screening, but adds weight to the argument for it. Sub-acute & non-specific presentation Individual often doesn’t realize: cannot rely on symptoms for the decision on screening “Healthy Migrant Effect” doesn’t apply Difficult to avoid TB Caused by bacteria Mycobacterium tuberculosis Spread by respiratory secretions Two categories of TB: Latent and TB Disease Latent TB TB Disease • Infected with M. tuberculosis • Also called “Active TB” • No signs (except reactive test) or symptoms--infected but not ill • Usually symptomatic • Cannot transmit tuberculosis • Can transmit to others Exposed individual TB Infection Latent TB Primary Disease eg pleural effusion, dissemination Reactivation (~10 – 15%) Natural eradication TB Disease XPTB (~30%) Pulmonary (~70%) Smear positive (advanced) Spontaneous cure (25%) Death (50%) Chronic (25%) Migration 400 - 200 years ago: Industrialization & urbanization led to TB rise: 25% deaths in Western Europe due to TB Grand migration through trade & colonization Wars, invasions in Europe &.... Now: Since ~1980 unprecedented human migration: 150 million people are long term residents of a country other than their COB (country of birth) Short term travellers 50 x as much Most migrants travel from high TB-incidence countries (> 40 / 105) to low TB-incidence countries (< 20 / 105) Tuberculosis Cases, United States, 1993-2009 2010 U.S.TB rate: 3.6 per 100,000 Are migrants specifically more at risk of TB reactivation? • Some specific groups clearly are e.g. refugees, migrants from war-torn countries • Age of TB reactivation migration coincide & Figure 5: Tuberculosis rate by Population subgroup and Age group, Australia, 2010 Australian-born Indigenous 60 Australian-born non-Indigenous Overseas-born 50 Notifications 40 30 20 10 • … but why does TB seem to reactivate just after migration? 0 – stress, Vit. D deficiency, increased 0-4 5 to 14 15 to 24 25 to 34 35 to 34 45 to 54 55 to 64 co-morbidity e.g. diabetes Year 65+ They achieved the Goal They screen in the source country before coming to USA, or to Australia, or to UK, or to Canada ! They invest to overseasTB screening in migration programs They invest and promote DOT treatment programs in the common countries of origin (born)-COB Who do they screen? The best predictor of TB is TB incidence in the COB Focus screening on high incidence countries Dependant on accuracy of rate estimates and correlation of country rate & rate in refugees Looking at the work done up to now could they do better? Discussion is about it, Targeting recognized risk factor e.g. past history, contact history, relevant co-morbidity: probably not. Close feedback loop (?) Targeting recognized risk groups from arrival country data (?) How do they screen? On the 24th March 1882, Robert Koch showed that TB was due to bacteria seen in sputum – Nobel Prize for Physiology or Medicine, 1905. On 8th November 1895, Wilhelm Röntgen, German Physicist, demonstrated x-rays – 1st Nobel Prize for Physics, 1901. How do we screen? Mobile x-ray unit, Gaziantep Find & Treat Service, Adana VSD, 2014 Tuberculosis Handbook, WHO, 1998: • “…two mandatory steps for detecting pulmonary TB: assessment of symptoms and microscopy examination.” • “… an optional second screening filter, chest radiography … alone should never be considered adequate…” X-rays in TB Chest x-ray: general – Sensitive: active pulmonary TB rarely not apparent Miliary or laryngeal TB potentially infectious but missed – Latent TB infection usually not seen (75%) – – Not specific - distinguishes poorly between: • TB and other diagnoses • Active and inactive TB – Protean manifestations – Changes often out of proportion symptoms – Lateral rarely adds anything, – Apical (lordotic) view can help CT rarely adds anything X-ray screening of migrants for TB Advantages: Easy for migrant and clinician Allows remote review Sensitive: rarely misses active pulmonary TB Lesions from LTBI can predict reactivation risk Disadvantages: Nonspecific: must be backed up by good microbiology Cost: financial, radiation How to screen ? sputum microbiology How do we screen? Smears ! Need both ! S&C Sputum smear has been the main stay: Diagnoses most important TB Culture now routine and required Smear too insensitive Drug resistance becoming more important QA improvements in culture important A Dedicated Induced Sputum Facility Improves Early Diagnosis of Tuberculosis Wong J, Waring J. 2014 • Audit: 21 months, dedicated induced sputum facility • Patients suspected of PTB, no spontaneous sputum • Induced sputum x 3, 6% hypertonic saline Results • n = 137 • 98 (72%) asymptomatic • 81 (60%) identified through x-ray screening • M.TB cultured in 33 (24%); – 26 (72%) with typical x-ray changes – 27 (82%) asymptomatic. • 97% positive results with first 2 samples • Minimal adverse events How do they screen – LTBI (?) Denholm J, McBryde E. Aust NZ J Public Health. 2014;38:78-82 • Screening for & treating LTBI in all new permanent immigrants would reduce TB incidence 34.5 / 106 to 16.9 – 23.8 / 106 • Need to screen 136 – 427 new arrivals for each case prevented Should we screen for LTBI? Debatable!! But USA asks screening for LTBI, age 2-15 TB in low incidence countries: LTBI reactivation after arrival Minimal missed active TB at the border Minimal evidence of transmission within low incidence countries Problems: Very large cohorts: > 50% applicants in high incidence countries Crude screening tests IGRAs no better: improved specificity not relevant and still doesn’t predict future active TB Crude treatment: most would receive potentially dangerous drugs un-necessarily Cost: many screened & treated for few cases avoided Why screening matters Migration is the crux of TB control in low incidence countries Australian-born Indigenous notifications 1400 Australian-born non-Indigenous notifications Overseas-born notifications 1200 86 – 88%TB cases are born overseas = 20 - 22 / 105 20 800 15 600 10 400 200 5 0 0 2002 2003 2004 2005 2006 2007 Year 2008 2009 2010 Rate (per 100,000 population) 25 1000 Notifications 30 2011 Data to 2009: Commun Dis Intell. 2012:36(1);82 2010 – 2011 data: personal communication, Dr Justin Waring, Medical Director, Western Australian TB Control Program, Chair, National TB Advisory Committee, Australia Tuberculosis cases, Europe, 2000 - 2010 Falzon D. et al. Italian J Pub Health. 2012;9(3) Tuberculosis rate, United States, 1993 - 2010 Falzon D. et al. Italian J Pub Health. 2012;9(3) Managing TB globally, not within borders New Engl J Med 2005;353(10):1008-20 Schwartzman et al. New Engl J Med 2005;353(10):1008-20 • Decision analysis model: multiple Markov processes • Est. active TB, TB-related death & associated costs in US • Done in relation to migrants (legal, undocumented & temporary visitors) from Mexico, over 20 years. Results 1. Current x-ray screening of legal migrants + usual TB control program: – 2. 35.4m migrants, 47 610 TB cases & 5245 TB deaths, costing $1.985 billion & $632 million respectively Adding LTBI screening to legal migrants – 401 fewer cases of TB, costing an extra $329 million Schwartzman et al. New Engl J Med 2005;353(10):1008-20 Results 3. Adding expansion of DOTS program in Mexico Cost to US = $34.9 million 2591 fewer cases & 349 fewer deaths, in undocumented migrants NET discounted saving over 20 years = $108 million Cost saving still if: … 88% initial investment doubled, US paid for allTB drugs in Mexico or decline in TB incidence in Mexico was less than projected Works by reduced LTBI in Mexican migrants once expanded DOTS improves TB incidence in Mexico New Engl J Med 2005;353(10): 1008-20