* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 5: Classification
Survey
Document related concepts
Artificial gene synthesis wikipedia , lookup
History of genetic engineering wikipedia , lookup
Point mutation wikipedia , lookup
Primary transcript wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Mir-92 microRNA precursor family wikipedia , lookup
Transcript
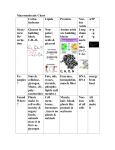
Molecular Biology EXAM STUDY GUIDE for FINAL MOLECULAR BIOLOGY MASTER LIST OF UNITS Unit 1: Scientific Method Unit 2: Chemistry Basics Unit 3: Organic Molecules Unit 4: Energy Unit 5: Cells and Cell Transport Unit 6: Classification Unit 7. Evolution Unit 8: Photosynthesis Unit 9: Respiration Unit 10: Cell Division Unit 11: Protein Synthesis Unit 12: Reproduction Unit 13: Genetics MAJOR THEMES OF BIOLOGY 1. Structure is related to function. ex. waterproofing on organisms is made of lipids which are nonpolar. ex. structures which absorb light are flat and thin to maximize surface area with the least amount of material 2. New properties emerge at each level of organization. Small changes in molecules can result in an enormous change for an organism. Diseases and disorders have a biological cause, often at the molecular or cellular level. ex. AIDS is caused by HIV ex. a single change in one nucleotide out of 1800 is the cause of sickle cell anemia. 3. Small changes in organisms over a long period of time can result in very large changes. ex. decisions that you make on a daily basis can have long term consequences on your health. ex. natural selection acting almost imperceptibly over hundreds of millions of years has created the wide range of species in existence today 4. Organisms inherit traits from their parents. These traits are made of DNA on genes in chromosomes. 5. Homeostasis is the idea that successful organism body systems are designed for self-regulation. Homeostasis means “staying the same.” Parameters that are maintained within limits include body temperature, blood pH, heart rate, glucose concentration, ion concentrations and blood pressure. 6. Energy cannot be created or destroyed, but it becomes less useful in the form of heat that dissipates. Matter is recycled within ecosystems. Ecosystems require energy that enters as light and leaves as heat. 7. Organisms are interdependent. Cellular respiration requires the products of photosynthesis; the reactants of photosynthesis are the products of respiration. Animals, including humans, require plants to live. 8. Living things have differences that determine their biological classification. However all living things are made of cells, have genetic material in DNA , and use ATP for energy. These two reflect a them referred to as Unity in Diversity. 9. Humans damage the environment more than all other species combined. We are the stewards of the land, soil, air, water and living things on which we depend. Humans need to evaluate the long-term consequences of their actions. Too many people consuming products unsustainably is already having an irreparable affect on our ecosystems and our own health. Unit 1: Scientific Method SCIENTIFIC METHOD In science, problem solving is based on the interpretation of data. Steps in the scientific method: hypothesis, procedure, experiment, analysis and conclusion. In an experiment, there should be two groups, one control, and one variable. The variable group should have just ONE difference from the control; all other conditions should be IDENTICAL. A hypothesis is a guess about the outcome, a true theory is an explanation that has not yet been supported by all the evidence and a law is a principle that is so basic that scientists believe it will never be disproved. The independent variable is a measurable quantity that is causing change in the dependent variable. For example if you measured the height of a tree over time, time would be independent and the height would be dependent. Time allows the tree to grow larger, but changing the tree’s height would not influence time. Time is independent. If there were few test subjects in an experiment, there would be no way to differentiate between changes occurring from natural changes and changes caused by the intended variable. Likewise, if there are many, many organisms, and there is a major difference between the two groups, then you know that the variable most likely had an effect. The experiment also has to have an actual measurable data. For example, although body weight, hear rate and body temperature are measurable, “health” would NOT be an example of measurable data. MEASUREMENT AND NOTATION Metric system units are based on powers of 10. length - millimeter (mm); centimeter (cm); decimeter (dm); meter (m) area - determined by multiplying two length measurements; measured in square metric units volume – capacity; can be measured in cubic metric units or liters; are 1000 milliliters in a liter mass - measured in grams; 2.2 pounds equals one kilogram; one mL of water has a mass of one gram density – density is mass divided by volume; water has a density of one gram per mL concentration – measures how much solute is dissolved in the solvent; measured using the mass of the solute per volume of the solvent energy - measured in calories and Joules temperature - measured in degrees Celsius or Fahrenheit; water boils at 212ºF or 100º, and freezes at 32ºF or 0ºC specific heat capacity – a measure of how many calories needed to raise the temperature of a substance Unit 2: Chemistry Basics o o o o o o o o o o o o there are about 100 types of atoms called elements protons and neutrons found in nucleus; electrons found in the orbital (clouds, energy levels, shells) for an atom to be stable, must have 8 electrons in outer orbital (fill the orbital) molecules are multiple atoms put together (e.g. water) compounds are molecules put together elements essential to living things (CHNOPS) = Carbon, Hydrogen, Nitrogen, Oxygen, Phosphorus, Sulfur ions have too many or too few electrons (positive or negative charge) atomic mass determined by neutrons + protons atomic number = the number of protons; # of protons = # of electrons in uncharged atoms isotopes have different numbers of neutrons: ex. Caron 12 & Carbon 14 Isotopes are unstable, may release energy or particles in the form of radiation measuring released particles allows tracking of certain substances through an organism (e.g. phosphorus) CHEMICAL BONDS Covalent Bonds - electrons are shared equally b/n atoms - can be single, double, or triple bonds (6 electrons shared in triple) Polar Covalent - occur b/n hydrogen and oxygen of the same molecule - electrons are shared but not equally - oxygen has slight negative charge and hydrogen has slight positive charge Hydrogen Bonds- occur because of charges on separate molecules - opposite charges on different molecules attract - hydrogen bonding occurs Ionic Bonds - electrons are transferred from one atom to another to fill outer orbitals of both PROPERTIES OF WATER o Cohesive Properties of Water (all caused by hydrogen bonds) o Surface tension - hydrogen bonding at the surface Detergent & surfactant (in lungs) break surface tension o High Specific Heat Capacity - water requires a lot more energy to heat compared to other liquids limits changes in body temperature specific heat capacity = # of calories required to heat 1 g by 1 ° C specific heat capacity of water is exactly 1 calorie / ( °Celsius x gram) water stabilizes surrounding environment temp (water absorbs or releases heat without large change in temp) o High Heat of Vaporization - lot of energy required to boil water and release heat sweating helps to cool the body down o High Heat of Fusion - energy is removed to freeze water to melt, a lot of energy is added (hydrogen bonds have to be broken- takes a lot of energy) o Expansion When Freezing - solids usually sink in liquids; water floats as ice ice floats b/c its less dense, molecules farther apart- more air trapped ice provides insulation in lakes; it is sprayed on oranges to keep them from freezing o Adhesive Properties of Water o Capillary Action - water climbs up the inside of thin tubes the thinner the tubes, the higher it rises (capillary tube used in blood samples) causes the meniscus in a graduated cylinder (curve of water) moves water a short way in trees o Good Solvent - water dissolves things NOT a universal solvent - dissolves substances that have a charge: polar or ionic ex. glucose is soluble in water water ionizes - can form H+ and OH- ions ionization is measured by the pH scale - acid and base o Chemical Reactions o equation - symbolic representation of a chemical reaction left- reactants; right - products arrow shows direction of the yield o chemical reactions used to convert energy and to make new molecules activation energy is the energy input required to start the reaction amount of activation energy needed depends on pH, temperature and enzymes enzymes - lower the activation energy Unit 3: Organic Molecules 1. Organic Molecules organic = made with carbon atoms 4 types: protein, carbohydrates, lipids, nucleic acids 2. Proteins H amine | acid H2N-C-C=O | \ R OH made of amino acids 20 dif. types of amino acids (8 essential not made by body) proteins are polymers of amino acids amino acid structure (see above) amino acids come in right handed form or left handed form living organisms have L-form, once dead become D-form (racemization) two amino acids linked together create peptide bond putting any organic molecules together is called dehydration synthesis or condensation reaction 3. Enzymes enzymes that digest proteins are protease primary structure is order of amino acids (determine sequence of DNA) secondary structure is shape that string of amino acids assumes (alpha helix, pleated sheet, globular) tertiary structure is a combination of helixes sheets and globs heating and changes in pH can denature shape of proteins quaternary structure develops when several tertiary polypeptide are joined to make one functional protein protein functions – transport across cell membrane, collagen and elastin, histones, tubulin, keratin, enzymes used to break things down, build things, and change shape end in “ase” (ex. lactase) acts on the substrate of the molecule cause reactions to occur by reducing the amounts of activation energy needed are proteins and are made of amino acids inhibitors (competitive or noncompetitive) prevent the enzyme from functioning 4. Nucleic Acids make up DNA which contains genes phosphate nitrogenous base genes carry code for the order of amino acids RNA copies the info from DNA sugar RNA acts as directions (deoxyribose) nucleotide structure (shown at right) 4 types of nucleotides based on which base they have; bases: A, T, G, C adenine, thymine, guanine, cytosine when bases pair they make hydrogen bonds RNA has uracil instead of thymine bonding pattern A – T, G – C chromosomes are made of DNA which make up genes which determine traits and behaviors DNA makes enzymes that make everything else 5. Carbohydrates 2 types: sugars and starches basic formula: [C(H20)]N (carbon and water) monosaccharides are sugars and have 3 to 6 carbons and an oxygen atom in a ring simple sugars like fructose are made by plants sugars are broken down during glycolysis disaccharides are two monosaccharides sucrose = table sugar, lactose = milk sugar, maltose = malt sugar people who lack lactase are lactose intolerant polysaccharides: starch, cellulose, glycogen, chitin two carbohydrates found in plant cells: cellulose and fructose 6. Lipids contain carbon, hydrogen, oxygen lipids are nonpolar, don’t mix with water two types: triglycerides and cholesterol trigylcerides lipids - fats, oils, waxes. Made from one glycerol and 3 fatty acids fatty acid - long chain of carbon and hydrogen atoms with a carboxylic acid group length of fatty acid determines whether lipid is oil wax or fat waxes are solid, oil is liquid and fat is in between at room temp double bonds cause lipids to be unsaturated have highest calories per gram of all organic molecules phospholipids are similar to triglycerides but have one phosphate and 2 fatty acids polar end is hydrophilic, non-polar is hydrophobic in cell membrane cholesterol needed for cell membranes can block arteries and blood vessels processed foods, (like potato chips), certain dairy products, and meat all have lipids 7. Biochemical Reactions hydrolysis- takes molecules apart and inserts water dehydration - molecules are put together and a water molecule is formed as one of the products Unit 4: Energy 1. Energy and Nutrients - Free energy starts the work for animals - Free energy is the energy that becomes available when there is a chemical reaction - Chemical energy is a type of stored energy where organism get it from organic or inorganic nutrients - Living cells need constant energy to DO WORK - Heterotrophs obtain energy from other organisms living or dead - Autotrophs can produce there own energy by organic compounds from inorganic materials - Photosynthesis captures some of the chemical energy in inorganic compounds - Heterotrophs obtain there energy through food 2. Energy and Ecosystems - producers, consumers, decomposers, food web, abiotic, biotic factors, ecosystem, habitats, and biosphere are all key words for this concept of energy. 3. Energy Conversions - total energy is constant but in different forms - total energy in the universe remains the same an never goes away - energy cannot be created or destroyed, just changed in form and or location - living things cannot create their own energy, but must obtain it from an outside source - energy then gets converted into free energy 90% of energy is released into heat 4. Energy and Entropy - maintaining organization in all living systems is required by energy - in most ecosystems energy flows from the sun to producers to consumers to decomposers - producers do not use all light energy absorbed - all chemical reactions are not 100% energy efficient 5. Energy transfer and ATP - the energy carrier is ATP - ATP is the nucleotide containing an adenine, ribose and three phosphate groups - ATP is constantly being used, when used up it is turned into ADP Unit 5: Cells and Cell Transport 1. Eukaryotic v. Prokaryotic - Eukaryotes have a nucleus, prokaryotes do not - Eukaryotic cells have organelles with membranes, prokaryotes do not they only have ribosomes - Eukaryotes have chromosomes in pairs, Prokaryotes have single chromosomes - Eukaryotes have streaming in cytoplasm, Prokaryotes do not - Eukaryotes use mitosis during cell division, Prokaryotes do not - Eukaryotes have a cytoskeleton, Prokaryotes do not - Eukaryotes cell walls may have cellulose or chitin, Prokaryotes may have peptidoglycan - All of the kingdoms are Eukaryotic except for the Monera (bacteria) kingdom - Eukaryotes are more complex than Prokaryotes 2. Animal Cells - Cell Theory: all cells come from preexisting cells - Plasma membrane: thick, 2 lipid layers, proteins embedded - Nucleus: controls cell, contains most of genetic DNA - Cytoskeleton: provides shape, organization and movement, directs Eukaryotic cells to make different shapes, made of proteins - Lysosome: site of digestion - Centrioles: form organizing points for mitotic spindles in protista and animal cells - Mitochondrion: Where cellular respiration occurs - Endoplasmic Reticulum (ER): site of protein synthesis - Golgi Apparatus: modifies, sorts, and packages macromolecules in vesicles for delivery - Cytosol: Fluid- material surrounding organelles and catalyze cellular reactions 3. Plant Cells - Cell wall: thick, formed by living plant cells - Plasma membrane: thick, 2 lipid layers, proteins embedded - Nucleus: controls cell, contains most of genetic DNA - Cytoskeleton: provides shape, organization and movement, directs Eukaryotic cells to make different shapes, made of proteins - Mitochondrion: Where cellular respiration occurs - Endoplasmic Reticulum (ER): site of protein synthesis - Golgi Apparatus: modifies, sorts, and packages macromolecules in vesicles for delivery - Cytosol: fluid material surrounding organelles and catalyze cellular reactions - Chloroplast: thick, is where photosynthesis occurs - Vacuole: stores nutrients and waste products, is a large vesicle, it contains water and digestive enzymes 4. Diffusion & Osmosis - The movement of molecules from an area of greater concentration into areas of lesser concentration until concentration is evenly spread out - Diffusion is a spontaneous process, it has random distribution of molecules - Molecules are in constant motion and keep moving until evenly distributing - Osmosis is the movement of water across a selectively permeable membrane - Water diffuses down its concentration gradient - Water concentration is greater outside of the cell - Water diffuses into the cell and the cell swells - Osmosis usually causes animal cells to burst if too “diffused” - Plant cells swell but do not burst because of its’ rigid cell wall 5. Other Transport Mechanisms - Passive Transport: substances move down their concentration gradient through the appropriate transport proteins - Active Transport: substances move through transport proteins against their concentration gradient (low to high concentration) 6. Systems - Necessary for 1: Division of labor among cells 2: Individual cells cannot work together without regulation and coordination. 3: majority of cells are not in direct content with outside environment. Unit 6: Classification - Carolus linnaeus invented modern taxonomy, he wrote the book Systemae Naturae - seven levels of classification : kingdom, phylum, class, order, family, genus, species (kids playing catch on freeway get smashed) - human classification: animally, chordata, mammalia, primate, hominidae, homo, Homo sapiens - two organisms are the same species if they breed in nature and produce fertile offspring - the five kingdoms are: Animalia, Plantae, Fungi, Protista, Monera - Animalia: eukaryotic, multi-cellular, heterotrophic - Plantae: eukaryotic, multi-cellular, autotrophic - Fungi: eukaryotic, multi-cellular, heterotrophic - Protista: eukaryotic, single cell, autotrophic and heterotrophic - Monera: Prokaryotic, single cell, autotrophic and heterotrophic - three phyla of Monera ~archaebacteria - methanogens – anaerobic, chemosynthetic, produce methane - thermoacidophiles – chemosynthetic, live in hot habitats - halophiles – salty environments ~cyanobacteria – photosynthetic, contain chlorophyll, gives them the blue-green color ~eubacteria – heterotrophic, cause disease - shapes: coccus = round, bacillus = rod, spirilium = spiral - beneficial roles of bacteria: create antibiotics, sewage treatment, aid digestion - harmful roles of bacteria: spoilage of food, diseases - know how viruses replicate - retro virus injects RNA instead of DNA - geographical isolation prevents species from interbreeding - steps in plant evolution 1) algae (aquatic) 2) move onto land (mosses, liverworts) 3) develop stems (ferns, club moss, horsetails) 4) develop seeds (conifers, ginkgoes, gymnosperms) 5) develop flowers (dicots, monocots) Unit 7. Evolution A. Atoms, Isotopes, and Radioactive Decay - organisms are made of cells - the stability of an atom, its radioactivity, depends on the ratio of neutrons to protons - a half-life is the time it takes for half of a radioactive isotope to decay - radioactive dating is important because you can determine how old fossils B. The Origin of Life - Earth is 4.6 billion years old, and the universe is about 9 billion years old - conditions on early Earth included severe weather, volcanoes, earthquakes, no atmospheric oxygen - life on Earth began from 1) Creation or 2) extraterrestrial life came to earth or 3) evolution from primordial soup (heterotroph hypothesis) / life came from non-living things - Stanley Miller’s experiment showed that amino acids could be form from H2O, CH4, N2, NH3, H2, and CO2 by recreating the conditions of early earth - things necessary for pre cells: membrane, DNA or RNA C. Darwin’s Theory - prior to Darwin, people believed the Earth was made in 6 days - also people believed that species were unchanging and always existed that way - small changes eventually add up to very large changes over millions of years - Lamarck’s ideas: 1) organisms inherit traits 2) individual organisms develop traits they need 3) organisms are compelled to evolve into a “higher” organisms 4) acquired characteristics - only correct thing about Lamar’s ideas was that organisms inherit characteristics from their parents - Darwin’s sailed on the Beagle to the Galapagos islands; animals on island evolved differently than similar organisms on the mainland D. Evidence of Evolution – all involve comparing organisms Archeology: fossils show that certain organisms existed at certain times and not others Biochemistry: compares DNA or proteins of different organisms to show how closely related they were Artificial selection: humans can direct evolution by breeding certain organisms Biogeography: ex. dinosaurs on Pangaea vs. mammal distribution on current continents Anatomy: analogous, homologous, vestigial Embryology: embryonic similarity shows how closely related organisms are Unit 8: Photosynthesis 1. Joseph Priestly: conducted experiments with mice, plants and candles in air-tight containers 2. Jan Baptiste Von Helmont: showed that plant weight comes from water but NOT soil 3. Calvin Cycle: named for its discoverer that involves the enzyme rubisco and that causes carbon fixation during photosynthesis; uses chemical energy previously converted from light energy 4. Light Reactions: energy capturing reactions in photosynthesis 5. Greenhouse Effect: warming of Earth due to atmospheric accumulation of carbon dioxide, which reflects infrared radiation that is absorbed as heat 6. Rubisco: an enzyme that catalyzes the initial incorporation of carbon dioxide in the Calvin Cycle. 7. Photosynthesis is vital; it is used in everything from the materials used in clothing, to powering automobiles and to supply oxygen. 8. 6CO2+ 6H20 C6H12O6+ 6O2 : equation for photosynthesis 9. Glucose is the product of photosynthesis. 10. The rate of photosynthesis can be determined by the amount of oxygen released or carbon dioxide consumed. These factors affect the rate: light intensity, temperature, and the concentrations of carbon dioxide and oxygen. 11. An increase in carbon dioxide concentration increases the rate of photosynthesis to a maximum point. 12. Guard cells are the cells that open and close the stomata. 13. Carbon dioxide, NADPH, and ATP are three molecules required for the Carbon Cycle to occur. 14. Thylakoids are the solar panels of the cell. 15. Chlorophyll is the main pigment of a photosynthesizing leaf. 16. ADP and NADP are two molecules that carry energy trapped by light reactions. 17. Sunlight is the source of energy for photosynthesis. 18. A vein is a leaf structure that contains the xylem and phloem. *** Xylem UP! Phloem DOWN! *** 19. Vascular tissue transports water, minerals, and nutrients. 20. Chlorophyll breaks down in autumn when the apparent color of the leaves change. 21. The stomata is where the plant breathes. 22. Chloroplasts are the organelles in which photosynthesis occurs. 23. Leaf cells, which are specialized to absorb light, are in the palisade layer. 24. Waxy cuticle is a waxy layer o the outside of leaves for waterproofing. 25. Chromatography is a process for separating pigments. 26. ATP is the energy currency of a cell. 27. Transpiration is a process in which plants lose water. Unit 9: Respiration 1. Types of Respiration: aerobic (with oxygen) and anaerobic: (without oxygen), occurs in cytoplasm only 2. ATP: the energy currency of all cells; structure: Nucleotide with 3 phosphates. (1 Sugar & 1 base) 3. Phosphorylation: Adding a phosphate to ADP to create ATP. 4. Organisms that use cellular respiration are organism: have mitochondria, breath oxygen, & create glucose. 5. Equation Glycolysis: C6H12o6 = 2pyrutate 2ATP 2NADH 6. Formation of ATP: ADP + P = ATP 7. NADH & FADH: carry hydrogen to the electron transport. 8. Three Stages of Cellular Respiration: GLYCOLYSIS, KREB CYCLE, and ELECTRON TRANSPORT 9. Formation of NADH: NAD + H = NADH 10. Pyruvate Pathways: go into fermentation & enter mitochondria 11. Krebs Cycle: CO2 ,NADH and ATP are released. 12. Hydrogen atoms deposited for ETS in the mitochondria in the inner membrane space. 13. Electron Transport: 32 ATP and 6 H2O created. 14. Why you need oxygen: O2 is required by aerobic respiration produces much more ATP. Oxygen picks up the hydrogen atoms at the end of electron transport. During high levels of activity without O2, the H+'s would build up and pH would plummet to lethal levels. 15. Person can survive without oxygen for 4-6 minutes. 16. Metabolic Reactions: synthesis, decomposition 17. know the cellular respiration diagram Unit 10: Cell Division 1. Cell cycle A. Interphase includes G1 phase, S phase, & G2 phase B. G0 phase - cell does not divide or grow, it is out of the cell cycle B. S Phase I. Accumulation of trigger proteins stimulates a cell to move into Synthesis phase from G1. II. Number of Organelles doubles III. Chromosomes replicate becoming X’s C. G2 Phase: cell continues to grow D. Mitosis I. Prophase - nuclear membrane disappears and spindle fibers form II. Metaphase - centrioles at opposite sides of cell, chromosomes line up on equatorial plane III. Anaphase - chromatids are pulled to opposite ends by spindle fibers IIII. Telophase - Nuclear Membrane reforms and cell splits forming two diploid daughter cells IV. Cytokinesis - partly during telophase, division of whole cell, splits forming two diploid daughter cells that are exactly the same In animals cells- cell membrane constricts forming a grove called the cleavage, cells separate In plant cells vesicles produced by golgi bodies from a mid line, vesicles than fuse to make a cell plate, plate attaches to cell wall, pectin fills in the layer between the two cells, and finally the cell adds in a secondary cell wall containing cellulose to split V. Reason for Mitosis is growth, healing, and development 2. Meiosis A. DNA replicates, chromosomes become X’s B. Chromosomes line up on equatorial plane in pairs. Crossing over can occur. C. Pairs split up, centromeres do NOT D. The cell splits E. Chromosomes line up again on equatorial plane as single X’s F. Centromeres split, as in mitosis G. Cells split forming four haploid daughter cell which are different from each other H. This whole process forms haploid gametes for sexual reproduction 3. Binary FissionA. Used by prokaryotes to make more cells B. A single circular chromosome of double stranded DNA is replicated and the plasma membrane splits 4. Cancer A. Proto-oncogene keeps cells from dividing to quickly. If genes mutate to oncogenes and control of division speed is lost, cancer results B. The crab is the symbol for cancer Unit 11: Protein Synthesis A. Transcription The “letter sequence of the genetic code is copied from DNA to RNA in the process of transcription -A–U RNA polymerase binds to “promoter” site of DNA and copies a gene. - T–A U is matched with A in the RNA because there is no T in RNA -G–C-C–GB. RNA Processing RNA must be processed. Useless parts called “introns” are removed in a process called splicing. An “mG cap” and a “polyA tail” is added to protect the RNA from enzymes outside the nucleus. mRNA = messenger RNA. Carries DNA information and amino acid sequence to the ribosome. tRNA = transfer RNA. Carries amino acids to the ribosome. Has an anticodon to correspond to the mRNA rRNA = ribosomal RNA C. Genetic Code Three letters make one word, or codon. (ex: A-C-G) Each amino acid is represented by a codon. (ex: UUC = phenylalanine) With four letters and 3 letters per word, 64 codons are possible. There are only 20 amino acids; multiple codons can represent an amino acid. The order of the codons on the gene determines the sequence of amino acids in the polypeptide. Each polypeptide can be considered a sentence. Special “start” and “stop” codes show when a polypeptide chain begins and ends. D. Translation Translation is putting amino acids in the right sequence to produce polypeptide chains. tRNA picks up amino acids and attaches to the mRNA at the ribosome with its anticodons that correspond to the codons on the mRNA. Unit 12: Reproduction A. Asexual Reproduction 1. disadvantage: lack of genetic diversity 2. advantage: only one parent is required. 3. Bacteria use binary fission to reproduce; single-celled eukaryotes use mitosis. 4. Plants reproduce asexually using both naturally and artificially (by human intervention.) 5. Sponges, hydra and flatworms reproduce asexually. 6. Bees reproduce asexually because drones grow from unfertilized eggs. 7. Every kingdom uses asexual reproduction. Every kingdom except Monera use sexual reproduction. B. Meiosis 1. Meiosis creates 4 haploid gamete cells. 2. Haploid have HALF the number of chromosomes that the diploid cells have. The somatic cells have the Diploid number, 2n, while the gametes have the haploid number, n. 3. Homologous chromosomes are pairs of chromosomes that have the same purpose with only minor differences. C. Flowers 1. Male flower components are collectively called the stamen that includes the anther and the filament. Female flower components are collectively called the Carpel which includes the Stigma Style and Ovary. 2. Pollination is when pollen goes from the Anther to the Stigma. 3. The pollen grain contains 2 Polar and 1 Tube nuclei. 4. The pollen creates a tube down to the nuclei. 5. The micropyle is the entry to the ovary 6. During double fertilization one of the polar nucleus bond to 7. The endosperm is unusual as it is a triploid. Its purpose is to feed the zygote. 8. The ovule and the ovary eventually becomes the fruit. 9. The three parts of the cell are the Endosperm, the Cell, and Zygote D. Animal Reproduction 1. Male gametes are much smaller and more mobile that female gametes. 2. Internal fertilization is advantageous as it increases the chance of fertilization as well as decreasing the number of sperm needed. 3. The sperm has a head that includes the acrosome and nucleus. The neck connects the head to the middle piece that contains the Spiral mitochondria. The tail is a flagellum. 4. Three mammals reproduction strategies: placental, monotreme and marsupial. The placental mammals contain the baby in a internal placenta which provides for the children. Monotremes lay eggs. Marsupials raise their children in pouches to maturity. E. Human Reproduction 1. Ovulation is the process of releasing eggs through the fallopian tubes and into the uterus. 2. Oogenesis is the creation of the eggs in the ovaries. 3. Pregnancy occurs around day 14 of the Cycle. 4. After fertilization the zygote embeds itself in the uterine lining and begins to develop. 5. A test tube baby is a child who was conceived in a test tube but developed in a uterus. Unit 13: Genetics 1. Gregor Mendel’s Principles (there are exceptions to each of these) a. Dominance: one version of a gene can mask or hide other versions of the gene b. Segregation: only one gene of each pair is given to the offspring by each parent c. Independent Assortment: the inheritance of one gene does not affect the inheritance of other genes 2. Punnets square simulate the possible results of meiosis and fertilization 3. Vocabulary: heterozygous, phenotype, allele, filial, true breeding, hybrid, and autosome 4. Patterns of Inheritance A. Dominance 1. simple dominance - genes in which one gene is dominant over the other ex. Bb, B is dominant over b 2. dominant does NOT mean the most common: ex. dwarfism and polydactyly (extra fingers and toes) are dominant traits 3. codominance - both genes are expressed (ex. antigens A & B are codominant) 4. incomplete dominance- only one intermediate trait is expressed ex. red and white parents: two pink offspring B. Linked Genes and Crossing Over C. Normal and Abnormal Sex Determination and Sex-linked Traits Sex-linked Traits: Hemophilia, Color Blindness, and Muscular Dystrophy XY- normal male XXY- Klinefelter Syndrome XX- normal female XYY- Super Male X- Turner Syndrome XY female- sterile female, no SRY gene D. Trisomy- three of the same chromosomes Non-disjunction- failure of chromosomes to separate during meiosis -results in cells with extra chromosomes Down Syndrome- extra #21 chromosome F. Multifactorial / Polygenic Traits: many genes control height, skin color, intelligence 5. Applications A. Karyotyping- used to sex determination (use white blood cells) B. Amniocentesis- determines sex and if child has Down Syndrome (used more often with women that bear children over the age of 40 C. Barr Bodies- found in normal cells of female mammals, come from one X which is turned off (ex. All Calico cats are female because of Barr bodies)