* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download ppt - Earth and Space Sciences at the University of Washington
Composition of Mars wikipedia , lookup
Geochemistry wikipedia , lookup
Physical oceanography wikipedia , lookup
Global Energy and Water Cycle Experiment wikipedia , lookup
Evolutionary history of life wikipedia , lookup
Blue carbon wikipedia , lookup
Biochemical oxygen demand wikipedia , lookup
Ocean acidification wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
History of climate change science wikipedia , lookup
Anoxic event wikipedia , lookup
History of Earth wikipedia , lookup
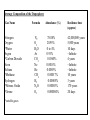
Average Composition of the Troposphere Gas Name Nitrogen Oxygen *Water Argon *Carbon Dioxide Neon Helium *Methane Hydrogen *Nitrous Oxide *Ozone *variable gases Formula Abundance (%) N2 O2 H2 O Ar CO2 Ne He CH4 H2 N 2O O3 78.08% 20.95% 0 to 4% 0.93% 0.0360% 0.0018% 0.0005% 0.00017% 0.00005% 0.00003% 0.000004% Residence time (approx) 42,000,000 years 5,000 years 10 days ~Infinite 4 years ~Infinite ~Infinite 10 years 3 years 170 years 20 days EVOLUTION OF THE ATMOSPHERE • Earth thought to have formed about 4.5 billion years ago: Atmosphere probably consisted of gases then abundant in the solar system -> hydrogen and helium. Most of these gases were lost to space • Over time a secondary atmosphere was formed: (Current atmosphere doesn't contain much hydrogen or helium). outgassing from cooling magma Volcanoes efflux: H20, CO2, SO2, N2, H2, Cl2 • Upon cooling of this prehistoric atmosphere: Water vapor condensed and precipitated to form oceans. Some carbon dioxide dissolved in droplets also precipitated out. • Eventually anaerobic bacteria developed some 3.5 billion years ago: Could survive in the absence of oxygen. Began the conversion of carbon dioxide to oxygen while removing Carbon Dioxide which is now stored primarily in carbonate rocks. Plankton and shellfish continue this process more effeciently. • As oxygen started to become abundant, some of it broke down by the sun’s radiation into atomic oxygen and eventually formed ozone . • Ozone absorbed most of harmful ultraviolet radiation to make Earth suitable for life. The atmosphere we know was produced by biological processes. Geological carbon cycle In the geological carbon cycle, carbon moves between rocks and minerals, seawater, and the atmosphere. Carbon dioxide in the atmosphere reacts with some minerals to form the mineral calcium carbonate (limestone). This mineral is then dissolved by rainwater and carried to the oceans. Once there, it can precipitate out of the ocean water, forming layers of sediment on the sea floor. As the Earth’s plates move, through the processes of plate tectonics, these sediments are subducted underneath the continents. Under the great heat and pressure far below the Earth’s surface, the limestone melts and reacts with other minerals, releasing carbon dioxide. The carbon dioxide is then re-emitted into the atmosphere through volcanic eruptions. (Illustration by Robert Simmon, NASA GSFC) Slow time scale - controls atmospheric carbon dioxide on time scales of hundreds of millions of years Biological/Physical carbon cycle - shorter than geologic cycle Land plants ~ 50 years atmosphere ~ 4 years soils ~ 25 years Fossil fuels ~ 650 years oceans ~ 100s to 1000s years carbonates ~ 150 years Addition of O2 to the Atmosphere Today, the atmosphere is ~21% free oxygen. How did oxygen reach these levels in the atmosphere? Revisit the oxygen cycle: * Oxygen Production o Photochemical dissociation - breakup of water molecules by ultraviolet + Produced O2 levels approx. 1-2% current levels + At these levels O3 (Ozone) can form to shield Earth surface from UV o Photosynthesis - CO2 + H2O + sunlight = organic compounds + O2 - produced by cyanobacteria, and eventually higher plants - supplied the rest of O2 to atmosphere. Thus plant populations-> * Oxygen Consumers o Chemical Weathering - through oxidation of surface materials (early consumer) o Animal Respiration (much later) o Burning of Fossil Fuels (much, much later) Throughout the Archean there was little to no free oxygen in the atmosphere (<1% of presence levels). What little was produced by cyanobacteria, was probably consumed by the weathering process. Once rocks at the surface were sufficiently oxidized, more oxygen could remain free in the atmosphere. During the Proterozoic the amount of free O2 in the atmosphere rose from 1 - 10 %. Most of this was released by cyanobacteria, which increase in abundance in the fossil record 2.3 Ga. Present levels of O2 were probably not achieved until ~400 Ma. Vertical structure of atmospheric pressure Atmospheric temperature: vertical structure Vertical structure as a function of latitude Venus Mars The Ocean: composition • Dissolved salts comprise 3.5% by volume of sea water • Originate from weathered rocks, volcanic, and atmosperic sources Composition of sea water remarkably uniform and constant over time Density of ocean water varies by < 7% Variations of density as a function of salinity and temperature (T,P,S) (T,P,S) 1000kgm3 Vertical structure of ocean in different latitudes