* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Pharmacology of Local Anesthetics
Survey
Document related concepts
Transcript
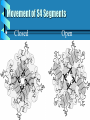
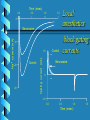
Pharmacology of Local Anesthetics John Yagiela, DDS, PhD UCLA School of Dentistry Pharmacology of Local Anesthetics History Definition Physical properties Mechanism of action Pharmacokinetics Adverse effects History 500’s Coca leaves first used by Peruvians for psychotropic properties 1850’s Cocaine isolated, hypodermic needle developed 1884 Sigmund Freud studies the effects of cocaine History (2) 1884 Carl Koller introduces cocaine into medical practice 1884 Local anesthesia used in dentistry by Halsted and Hall 1905 Procaine synthesized by Einhorn History (3) 1921 Cartridge syringe marketed by Cook 1947 1948 1959 Aspirating syringe developed Lidocaine marketed Disposable needle introduced Definition Local anesthetics are drugs that reversibly depress nerve conduction. "Caine" local anesthetics act more selectively than other agents. Physical Properties (structure) Ester: R 1—COO—R 2 —N R3 R4 Amide: R 1—NHCO—R 2—N R3 R4 R 1 — Lipophilic aromatic residue. R 2 — Aliphatic intermediate connector. R 3, R 4 — Alkyl groups, occasionally H. Constitute with N the hydrophilic terminus. Example: H2 N— —COO—(CH 2) 2—N C 2H 5 C 2H 5 Exception: Benzocaine, which lacks a substituted amino group (Acid-base considerations) Most local anesthetics are weak bases, pKa 7.5-9.0. Usually prepared as a salt (e.g., with HCl) to increase stability, water solubility. When injected, 5%-40% is converted to the nonionized free base. R-NH+ R-N + H+ acid base base pH = pKa + log acid Alveolar mucosa H 2N O C 2H 5 COCH 2CH 2 N O H H 2N C2H5 COCH 2CH 2 N + C2H5 C 2H 5 Cationic acid Log Base = pH – p Ka Acid + H Nonionized base Lipoid barriers [1.0] (nerve sheath) (Henderson-Hasselbalch equation) Extracellular fluid Base Acid [1.0] * [3.1] Acid [2.5] For procaine (p K a = 8.9) at tissue pH (7.4) Nerve membrane Base = 0.03 Acid Axoplasm Base Mechanism of Action Axonal membrane • Local anesthetics interfere with propagation of the action potential by blocking the increase in sodium permeability during depolarization. Mixed nerve Membrane potential (mV) +20 0 -80 30 gNa -20 -40 1 20 gK 10 -60 0 2 Time (msec) 3 4 Ion conductance (mmho/cm 2) +40 Developing local anesthetic block Membrane potential (mV) 40 A B 0 C D –40 –80 0 1 2 Time (msec) 3 Movement of S4 Segments Closed Open Time (msec) 0.0 0.5 1.0 Local anesthetics 1.5 0 block gating currents -10 Control 1.5 -20 -30 Control Gating current (nA) Na + current (nA) Benzocaine Benzocaine 0 -1.5 0.0 0.5 1.0 Time (msec) 1.5 Mechanism of Action (2) Mixed nerve • Local anesthetics provide pain relief by blocking nociceptive fibers. Other fibers are affected as well. Sensitivity to local anesthetics depends on: fiber diameter, fiber type, and degree of myelination. Sensory modalities are affected in the following order: pain, cold, warmth, touch, and pressure. Critical length theory Compound AP (% control) Frequency dependent block 100 } 80 60 Tonic block } Phasic block 40 20 0 0 1 2 3 Minutes 4 0.0 Time 0.1 0. 0.3 2Seconds 0.4 0.5 Pharmacokinetics Absorption • Local anesthetics are absorbed when ingested. Some local anesthetics may be absorbed in toxic amounts after topical use. Absorption after an injection depends on drug solubility in lipid and in water, tissue vascularity and local anesthetic and vasoconstrictor effects on local circulation. Pharmacokinetics (2) Metabolism and excretion • Esters are hydrolyzed by plasma and liver esterases. Longer-acting esters are often metabolized more slowly. Sulfonamide therapy may be neutralized by PABA liberation. Patients with altered pseudocholinesterase activity may be highly sensitive to these drugs. • Amides are metabolized in the liver. Patients with severe hepatic damage or advanced congestive heart failure may be unusually sensitive to these drugs. Some amides are partially excreted unchanged in the urine. Local anesthetic metabolism Ester Hydrolysis Hydrolysis CH 3 Amide Hydroxylation and conjugation O NHC CH R1 R2 R3 N R4 N-dealkylation (and cyclization) Adverse Effects Side effects • CNS toxicity—Entry of local anesthetics into the brain depression of CNS pathways. The clinical picture may include stimulation (e.g., excitement, disorientation, increased heart rate and respiration, tremors, and frank convulsions) if inhibitory neurons are affected initially. • CNS depression may cause hypotension, respiratory depression, unconsciousness, and death. Treatment includes supportive measures. Excitement and convulsions may be controlled with 5 mg dosess of diazepam or 2 mg doses of midazolam. Respiratory depression requires oxygen and possibly rescue breathing. Adverse Effects (2) • CVS derangement—High plasma titers may depress the cardiovascular system directly. Blood pressure may fall because of arteriolar dilation, myocardial depression, and/or cardiac conduction disruption. Treatment includes patient positioning, IV fluids, and vasopressors. Cardiac asystole will require CPR. Prevention of systemic toxicity—Limit the amount of drug employed. Use proper injection techniques. Adverse Effects (3) Allergy • Allergic reactions are rare, especially with amide local anesthetics. Urticarial rashes are most common, but more serious responses also occur. Mild skin reactions are treated with antihistamines; more serious sequellae require epinephrine. Adverse Effects (4) Syncope • The most common side effect of dental injections. Must be treated promptly since it may be dangerous in its own right and has to be differentiated from anaphylactic shock. Adverse Effects (5) Local toxic reactions • Selective destruction of skeletal muscle fibers. Epithelial damage from topical preparations. Local necrosis from vasoconstrictor actions.