* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Accessory pathways during normal and abnormal cardiac
Cardiac contractility modulation wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Coronary artery disease wikipedia , lookup
Heart failure wikipedia , lookup
Rheumatic fever wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Cardiac surgery wikipedia , lookup
Congenital heart defect wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
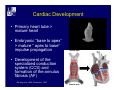
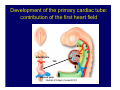
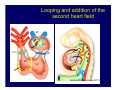
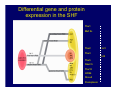
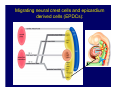
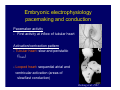
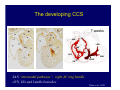
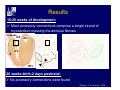
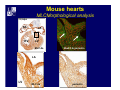
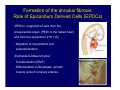
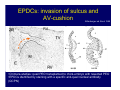
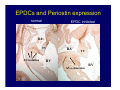
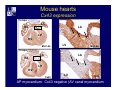
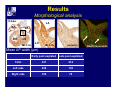
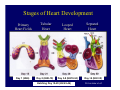
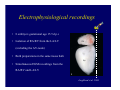
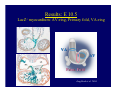
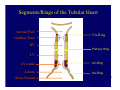
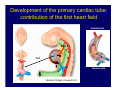
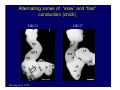
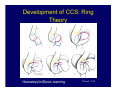
Accessory pathways during normal and abnormal cardiac development: relevance for WPW syndrome Nico A. Blom Leiden University Medical Center, Leiden, The Netherlands Physiology and experimental medicine symposium: cardiovascular performance in fetal and neonatal disease Hospital for Sick Children, Toronto, May 19, 2009 Development of the cardiac conduction system: relation with clinical arrhythmias • Research project of the departments of Anatomy and Embryology, Cardiology and Pediatric Cardiology of the LUMC • Focus: understanding of developmental background of clinical arrhythmias and conduction abnormalities • Morphological studies and embryonic EP studies (since 2006) • Ectopic atrial tachycardias, “internodal pathways”(Circ 1999, JCE 2003), Mahaim tachycardia (Circ Res 2005), AVN in CHD (Ped Res 2005), Sinus node disease (Circ 2007, Dev Dyn 2009), WPW syndrome (Circ 2007, Circ 2008, Circ 2008) 1. Introduction on cardiac development 2. Embryonic electrophysiology/ development of the CCS 3. Accessory pathways during normal and abnormal heart development Cardiac Development • Primary heart tube > mature heart • Embryonic “base to apex” > mature “ apex to base” impulse propagation Arterial pole • Development of the specialized conduction system (CCS) and formation of the annulus fibrosis (AF) De Jong et al, 1992; Chuck et al, 1997 Venous pole Development of the primary cardiac tube: contribution of the first heart field 2nd arterial pole 1st venous pole Human 23 days; mouse E 8.5 Looping and addition of the second heart field SV AoS SAR LA 2nd RA VAR AoS AVR RV “LV” PR human 25 days;mouse E11.5 Differential gene and protein expression in the SHF Tbx1 Mef 2c Tbx2 Isl1 Tbx3 Id2 Tbx5 Nkx2.5 Tbx18 HCN4 Shox2 Podoplanin Migrating neural crest cells and epicardium derived cells (EPDCs): Embryonic electrophysiology pacemaking and conduction Pacemaker activity - First activity at inflow of tubular heart Activation/contraction pattern - Tubular heart: slow and peristaltic (ICa2+) - Looped heart: sequential atrial and ventricular activation (areas of slow/fast conduction) DeJong et al. 1992 Surface ECG in tubular/looping heart “Mature” ECG at HH16 despite absences AVN & HPS 12 13 14+ 15 QRS 14 16 p Embryonic electrophysiology “Mature heart” (chick HH>35, mouse >13.5 days): ⇒ rapid atrial and ventricular activation (INa+) ⇒ development of compact AV-node ⇒ maturation of His-Purkinje system ⇒ Coincides with ventricular septation Change in ventricular activation pattern: - base to apex (chicken <HH 33-35, mouse <13.5 days) - apex to base ( chick >HH35, mouse >13.5 days) Chuck et al. 1997 Development of the specialized CCS What is the origin of cells of the CCS ? - myocardial heart tube (first heart field)? - second heart field ? - non-myocardial cell populations (NC-cells) ? ⇒ Retroviral lineage tracing: myocardial origin of CCS Visualization of the developing CCS • Antigen expression - HNK-1/Leu 7 - Id2 - NCAM (anti-PSA) - etc.. • Transgenic animals - CCS-LacZ reporter mouse - MinK-LacZ reporter mouse - etc…….. The developing CCS 7 weeks SS SAN LBB LVV RVV His RBB RAVR CS SAN,“internodal pathways”, right AV ring bundle AVN, His and bundle branches Blom et al. 1999 Accessory AV pathways during normal and abnormal cardiac development relevance for the pathogenesis of WPW syndrome Wolff-Parkinson-White syndrome • Incidence 0.15% (0.07 % in children) • Accessory atrioventricular pathway (AP): substrate for ventricular preexcitation and atrioventricular reentrant tachycardia • Concealed WPW (retrograde conduction only): 25% adults, 75% of the neonates • Majority no familial involvement, • Pathogenesis unknown • Minority of cases are inherited as single gene disorder: PRKAG2 gene mutation,others Öhnell 1943 Accessory pathways in the fetus and neonate • Supraventricular tachycardias relatively common in fetuses and neonates • >90% are AVRT • Fetal/neonatal AVRT can be life-threatening (fetal hydrops, severe forward failure) • Two-third remain free of symptoms without medication after one year Accessory pathways during cardiac development Formation of the annulus fibrosis: - Contribution of endocardial atrioventricular cushions on the luminal side and inward migration of AV sulcus tissue - Bone morphogenetic protein (BMP) signaling, Epicardium derived cells (EPDCs), Periostin Summary of three studies 1. Normal human heart development: (immuno) histochemical studies 2. Normal quail and mouse heart development: electrophysiological experiments, immunohistochemical studies 3. Effect of EPDC inhibition in quail hearts: electrophysiological experiments ,immunohistochemical studies Human embryos/fetuses •44 human hearts: 4 weeks of development – 2 days postnatally •Myocardial markers (HHF-35, MLC2a), Fibrous tissue markers (fibronectin, collagen VI, periostin) •4-6 weeks: All myocardium continuous at primitive AV canal •6-10 weeks: Mainly broad myocardial continuities (post-septated hearts) 7,1 weeks LA LA 35% 20 VS LV RV RV 45% % VS LV MLC-2a Hahurij, Circulation 2009 Results 10-20 weeks of development: ¾ Most accessory connections comprise a single strand of myocardium crossing the annulus fibrosis 16 weeks RA RA AO RV 67% 16 RV rAVR 17% % RF+ 20 weeks-birth-2 days postnatal: ¾ No accessory connections were found Hahurij, Circulation 2009 Accessory connections related to the AVN 14,2 weeks RA HIS * LBB LA TV MV VS RV AVN LV VS RF+ Hahurij, Circulation 2009 ¾ accessory AV connections with nodal phenotype? Conclusion (1) • AF isolation is an ongoing process after the first trimester and APs remain present in normal human fetuses until late fetal stages • Clinical relevance? • Conducting properties? Accessory pathways in normal embryonic quail and mouse hearts RA Temperature 35,5 – 37 0C LVB Perfusion with oxygenated RVB Tyrode solution LVA EP recordings in normal quail (n= 80) and mouse embryos (n=42) during SR and RA pacing Mouse embryos: 13.5 –birth (postseptation early and late) Quail embryos: preseptational (HH 30-34) and postseptational (HH 35-44) Kolditz, Circulation 2007, Hahurij 2009 Methods Mean HR, AV conduction times Ventricular activation sequence • “base-to-apex” (base ≥ 1 ms earlier than apex) • “apex-to-base” (base ≥ 1 ms earlier than apex) • “concurrent” (base and apex <1ms) •Immunohistochemistry: α-MLC-2a α-Periostin, α-Cx43, αNkx2.5 •Location and size of APs at the developing Annulus Fibrosis LV base to apex activation Pre-septational heart HH 31 quail heart; base-to-apex LV-activation 2 μσ 200 mm/s Sinus Rhythm 1600 mm/s Apex to base activation pattern (post-septated heart) ΗΗ 39 quail heart; apex-to-base LV-activation LA LVB 9 μσ LVA 200 mm/s RA pacing 120/min 1600 mm/s Example of premature base activation in a late post-septational heart HRA pacing HH 39; LV-apex-to-base, RVB first Ω atrial capture 8 ms 1 ms Quails Electrophysiological recordings Apex-first Group A HH 30-44 Group B HH 35-44 Base-first Concurrent RVB-first LVB-first 7 % (n = 1) 20 % (n = 3) 40 % (n = 6) 33 % (n = 5) 49% (n = 32) 17% (n = 11) 8 % (n = 5) 26% (n = 17) 51% (n = 33) of post-septated hearts shows premature base activation ! Quail heart Right posteroseptal AV-ring (HH 38) ibr osi s Immunohistochemistry – MLC2a an n ulu sf RA RA LV RV MLC2a 4x s u l nu n a sis o r fib RV AV myocardial continuity MLC2a 40x Quail hearts Immunohistochemistry – MLC2a & periostin MLC2a periostin RA LV + RV 4x periostin myocardial cell fibroblast cell Mouse hearts MLCMorphological analysis 13,5dpc RA RV LA LV MLC-2a Nkx2.5 & periostin LA LV MLC-2a periostin Mouse hearts Morphological analysis Mean AP width (μm) Early post-septated Late post-septated early vs late Total 347 214 P=0,035* Left side 238 138 P=0,000* Right side 109 76 P=ns Hahurij et al 2009 Conclusions (2) • Transient APs are present during normal avian and mouse heart development and have antegrade conducting properties • Periostin may have an inductive role in formation of fibrous tissue of the AF Formation of the annulus fibrosis: Role of Epicardium Derived Cells (EPDCs) -EPDCs: outgrowth of cells from the proepicardial organ (PEO) to the naked heart and form the epicardium (HH >16) - Migration to myocardium and subendocardium -Epithelial-to-Mesenchymal Transformation (EMT) Tubular heart - Differentiation to fibroblasts, smooth muscle cells of coronary arteries PEO Epicardial Outgrowth SV cytokeratin HH17 proepicardial organ HH20 epicardium EPDCs: invasion of sulcus and AV-cushion Gittenberger-de Groot 1998 RA •Chimera-studies: quail PEO transplanted to chick-embryo with resected PEO •EPDCs identified by staining with a specific anti-quail nuclear antibody (QCPN) Role of EPDCs in formation of the annulus fibrosis? Mechanically inhibition of PEO outgrowth HH16 OT Ao RV inserted egg shell membrane Atria Pro-Epicardial Organ Wild type versus EPDC-inhibited hearts Ex ovo electrogram Kolditz , Circulation 2008 EPDC-inhibited heart: in ovo ECG: Ventricular preexcitation HH40 Wild type HH41 EPDC-inhibited Wild type vs EPDCinhibition AP volumes Large APs left and right lateral EPDCs and Periostin expression normal EPDC inhibited RA TV AV-isolation RA TV RV AV-connection RV Conclusions (3) • Functional APs are present during normal heart development in avians and mammals • “physiological” APs may serve as substrate for transient AVRT in the fetus and neonate • EPDCs play an important role in the formation of the annulus fibrosis • Periostin plays an inductive role in fibrous tissue formation • Abnormal migration of EPDCs may play a role in the pathogenesis of WPW syndrome ? Acknowledgments • Dept of Pediatric Cardiology, LUMC, The Netherlands Nathan Hahurij MD Regina Bökenkamp MD • Dept of Cardiology, LUMC, The Netherlands Denise P. Kolditz MD, PhD Martin J. Schalij MD PhD • Dept of Anatomy & Embryology, LUMC, The Netherlands Adriana C. Gittenberger-de Groot PhD Robert E. Poelmann PhD • Medical University of South Carolina, Charleston SC, USA Roger R. Markwald PhD Thank you for your attention Mouse hearts Cx43 expression 15.5dpc LA LA RA LV RV MLC-2a LV MLC-2a 15.5dpc RA LA LA LV RV Cx43 LV Cx43 AP myocardium: Cx43 negative (AV canal myocardium Results Electrophysiology Apex > Base 85BPM Base > Apex 77BPM Results Electrophysiology data Activation pattern Early post-septated HR 115±4BPM AV 80±17msec Late post-septated HR 95±27BPM AV 81±18msec Apex > Base 38% (n=11) 46% (n=6) Base > Apex 62% (n=18) 54% (n=7) LVB > Apex 7% (n=2) 31% (n=4) RVB > Apex 7% (n=2) 8% (n=1) Concurrent 48% (n=14) 15% (n=2) Results Morphological analysis 13,5dpc LA RA RV LA LV MLC-2a LV MLC-2a periostin Nkx2.5 & periostin Mean AP width (μm) Early post-septated Late post-septated early vs late Total 347 214 P=0,035* Left side 238 138 P=0,000* Right side 109 76 P=ns left vs right P=0,000* P=0,028* * Statistical test XXX Mouse hearts electrophysiology data Activation pattern Early post-septated HR 115±4BPM AV 80±17msec Late post-septated HR 95±27BPM AV 81±18msec Apex > Base 38% (n=11) 46% (n=6) Base > Apex 62% (n=18) 54% (n=7) Concurrent 48% (n=14) 15% (n=2) Stages of Heart Development Primary Heart Fields Day 1 (HH6) Tubular Heart Day 2 (HH8-10) Looped Heart Day 3-8 (HH10-34) Hatching Day 18-20 (HH 45-46) Septated Heart Day >9 (HH>35) Srivastana et al. Electrophysiological recordings • 8 embryo's gestational age 15.5 d.p.c • Isolation of RA/RV from the LA/LV (including the AV-node) • Both preparations in the same tissue bath • Simultaneous EGM recordings from the RA/RV and LA/LV Jongbloed et al. 2004 2 out of 8 embryo’s SR with 1:1 conduction RA 稀 RV 2:1 VA conduction LV 稀 LA Right Lateral AV-connection Jongbloed et al. 2004 “CCS-LacZ reporter mouse” Rentschler et al. 2001 CCS-lacZ Expression in Pulmonary Veins Jongbloed et al. 2004 Results: E 10.5 LacZ+ myocardium: AV-ring, Primary fold, VA-ring VA AV Prim. Fold Jongbloed et al. 2004 Results: E 11.5 Formation of myocardial groove and outgrowth of LacZ- myocardium at inflow-tract Jongbloed et al. 2004 Jongbloed et al. 2004 Results: E 13.5 Division of the primary fold tissue: 稀 Medial part: trabecula septomarginalis 稀 Lateral part: moderator band MB Jongbloed et al. 2004 Segments/Rings of the Tubular Heart ARTERIAL POL Arterial Pole Outflow Tract RV VA-Ring OUTFLOW TRA Primary Ring LV AV-canal Atrium Sinus Venosis AV-Ring PRIMITIVE ATR SA-Ring SINUS VENOSU HOMING AND ROLES OF NEURAL CREST CELLS outflow tract (OFT) migration OFT inflow tract (IFT) SMC differentiation IFT PNS RA myocardialisation induction conduction system (CCS) RV • • High expression of both Tbx5 and Nkx2.5 establishes a regionally restricted program of ventricular CS gene expression, including the Id2, Cx 40 and ANF promotors This program recruits cells into CS lineage, including cell cycle exit, adoption of fast conduction, expression of other CS markers Moskowitz,Cell, 2007 Development of the primary cardiac tube: contribution of the first heart field arterial pole 2nd OT “LV” A 1st SV venous pole Human 23 days; mouse E 8.5 Alternating zones of “slow” and “fast” conduction (chick) HH 23 DeJong et al. 1992 HH 27 Development of CCS: Ring Theory Hematoxylin/Eosin staining Wenink, 1976