* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 19, Coordination and Organometallic Compounds
Allotropes of carbon wikipedia , lookup
Bond valence method wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Cluster chemistry wikipedia , lookup
Hydroformylation wikipedia , lookup
Metal carbonyl wikipedia , lookup
Jahn–Teller effect wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Metalloprotein wikipedia , lookup
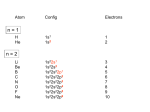
Greenwood & Earnshaw 2nd Edition Chapter 19 Coordination & Organometallic Compounds The Transition Elements All the elements arising from the filling of the 3d, 4d and 5d shells are metals and comprise Groups 3-12. As a group they are lustrous, deformable, have high electrical and thermal conductivities and mp/bp are high. Display multiple oxidation states which vary by 1 rather than by 2 as found in the main groups. They have an unparalleled propencity for forming coordination compounds with Lewis bases. Werner Theory: Primary Valency = Oxidation State Secondary Valency = Coordination Number; the number of Ligands directly bonded to the metal atoms. Ligands Mono- or Unidentate: Capable of forming one coordinate covalent bond with the metal atom, commonly through one orbital bearing a lone pair of electrons. Bidentate: Capable of forming two coordinate covalent bonds resulting in a ring, result is a chelate (Gk. claw). Tri- or Terdentate: forming three coordinate covalent bonds resulting in two rings. Tetra- or Quadradentate: forming four CC bonds. Penta- or Quinquidentate => 5 Hexa or Sexidentate => 6 1 Chelating Ligands Chelate Effect: A bidentate ligand, L-L, will commonly displace two unidentate ligands, L, in a kinetically labile coordination compound given both ligand sets have similar individual bond energies. Rationale: When one donor atom binds to the initial coordination site, the second donor atom is held in close proximity to the second coordination site. Chelating ligands forming five and six-membered rings are especially favored. Smaller rings induce ring strain. Larger rings suffer from entropy considerations. Chelating ligands can flexibly conform to the metal or can rigidly demand a specific coordination geometry. Stereochemistry & Coordination Number CN Coordination Number: L M L 2 Linear 3 Trigonal 4 4 Square Planar Stereochemistry: L L M L Tetrahedral L L M 5 L L 5 Trigonal Bipyramid L 5 Square Pyramid Pentagonal Plane unknown L L L L L L M L L L L L L M L M M L L L L L Stereochemistry & Coordination Number CN 6 Coordination Number: 6 Octahedron L Stereochemistry: L L M L 7 Capped Trigonal Prism 7 L L Capped Octahedron L L L L L L L L L M L L M L L L Pentagonal Bipyramid L L L L L 7 Hexagonal Plane unknown L L M L 6 Trigonal Prism M L L L L M L L L L L L L 2 Factors Affecting Coordination Numbers With dominant electrostatic forces a higher CN will be favored by high cation charge and low anion charge. The limiting CN is ultimately affected by the radius ratio of cation and anion. Where covalency is important charge is distributed, ligand polarizability will lower the CN required to satisfy a given cation. Pi (π) acceptor character of the ligand will again raise CN. The availability of empty orbitals for ligand donation affects CN. CN is lowest for TM with near-filled d subshells, heavier elements gps 11-12. However at the converse extreme for groups 3-4 CN is dominated by electrostatic interactions. Isomerism 1) Conformational or "Polytopal" vs Tetrahedral L Square Planar L L M Cis vs Facial (fac) B A A B M B B CN 4 or 6 3) Optical Isomers M A B A A B B A B M A B B B Meridional (mer) B B B A B B M A A B M B L Trans B A A L L L 2) Geometrical L M A B A M B B B M A B A B B Crystal Field Theory An Electrostatic Point Charge Model - absurd bonding but gave fundamental understanding of visible spectra. ∆ t = 4/ 9 ∆ h ∆h and ∆t = hνν, ν is in the range of visible light. ∆h Spherical Crystal Field ∆t Tetrahedral Crystal field Octahedral Crystal Field Free Ion Ligand Field Theory - Introduces some covalency into ligand-metal interactions. Valence Bond Theory - Allowed chemists to understand the bonding picture but said nothing about excited states. Molecular Orbital Theory - Gives a comprehensive bonding picture for Metal-Ligand interactions. 3 Complementary Colors Color Absorbed Violet Indigo Blue Blue-Green Green Yellow Green Yellow • • • • • • • • Color Observed Yellow-Green Yellow Orange Red Purple Violet Indigo Molecular Orbital Theory t1u* a1g* 4p 4s t1u eg* a1g ∆h eg eg t1u a1g six degenerate ligand donor orbitals 3d t2g t2g transition metal atomic orbitals eg sigma bonding t1u a1g molecular orbitals d2sp3 Molecular Orbital Theory Mid-Spectrochemical series ligands are sigma bonding only. π Donor Ligands: π Acceptor Ligands: eg. C O CN- eg. NH3 eg. Cl- eg* Weak Field Ligands are π-donors. The π-atomic orbitals are filled and low in energy they cause the ligand field splitting to diminish. ∆h ∆h ∆h t2g Strong Field Ligands are π-acceptors. The π∗-molecular π∗ orbitals are empty and high in energy they cause the ligand field splitting to increase. 4 Electron Configurations – Magnetism and Ligand Field Stabilization Energy eg* 3/5 ∆h < Epairing High Spin Complexes (Weak Field) 2/5 t2g d0 Ca+2 LFSE: d1 Ti+3 d2 Ti+2 d3 V+2 d4 Cr+2 d5 Mn+2 d6 Fe+2 d7 Co+2 d8 Ni+2 d9 Cu+2 d10 Zn+2 2/5 4/5 6/5 3/5 0 2/5 4/5 6/5 3/5 0 Low Spin Complexes (Strong Field) ∆h > Epairing Organometallic Compounds Hapticities the Hapto Nomenclature M η2 η1 M M M η3 η4 M M η5 bis-η η5 M M bis-η η2 η6 M M η7 η3 The nomenclature has been generalized to include other atoms such as N, O, P, etc. which occur in ligand systems besides carbon, eg. pyridine may be η6. η1 Ligands – Alkyl, Alkylidene, Alkylidyne Methyl – Methene - Methyne M H σ C 1e- π H H σ M π π M σ H 2e- H 3e- Two sets of p-bonds at right angles. π C H π C σ M C H π 5 Alkyl, Alkylidene, Alkylidyne In most carbyne complexes the metal-carbon triple bond is short and strong. Bond order-lengths are more normal here: In many carbene C(CH3)3 complexes the metalE C pCCW = 175E dCW = 176 pm carbon bond is only dCW = 194 pm H CH3 W slightly shorter than the pCCW = 150EE P CH3 metal-carbon single (CH3)3C C CH2 bond suggesting limited dCW = 226 pm double bond character. H pCCW = 125EE CH 2 C P Carbenes with N or O CH3 H in the alpha position are C(CH3)3 CH3 stabilized due to a more extended pi system. [Ta(η η 5-C5H5)(CH3)(CH2)] Carbene complexes are dTa-CH3 = 225 pm dTa-CH2 = 220.6 pm usually highly reactive species. Alkyls without β-hydrogen atoms cannot undergo βelimination (of alkene) reactions and thus are more stable. M-O Diagram – Carbon Monoxide C O C O σ∗ Antibonding orbitals very Carbon-like HOMO-LUMO gap π∗ 2p Lone pair of electrons on Carbon σ 2p * π 2s sp hybridization * MO mixing of s&p σ Lone pair of electrons on Oxygen too low in energy to be good donor. 2s Carbon Monoxide C O σ∗ C O π∗ The antibonding molecular orbitals extend more on the carbon side, a better π-acid, relatively low in energy. σ C The carbon lone pair is most available for σ-donation. π C O The strong pi-bond places most electron density on oxygen. O The sigma bond is also strong placing most electron density on oxygen. O The oxygen lone pair is a very poor donor. σ C 6 Metal - η1 Carbon Monoxide Bonds π∗ d O σ M O C C π Terminal C-O Bonding π σ M M O C π σ M Bridge C-O Bonding π Facial Bridge C-O Bonding M M Carbon Monoxide – Higher Hapticities a) b) c) O C M O C M O C M M M M M M Two versions of the unsymmetrical facial bridge bond to three metals. The unsymmetrical C-O bridge bond. a) The C-O is η1 to one metal atom and η2 to the other. b) The C-O is η1 to one metal atom and η2 to the other two metal atoms. The M(CO)M angle is ~90E. c) The C-O is η1 to two metal atoms and η2 to the third. The bis(η η1) bridge is symmetrical. Alkene and Alkyne Metal Complexes C M π∗ d π∗ d H H H C M σ σ C C H H η2-alkene - 2e donor H C H η2-alkyne - 2e donor d π M σ C H C M M η2-alkyne - 4e donor µ2,η2-alkyne - 4e donor 7 Alkene and Alkyne Metal Complexes R Ph3P R=H R C Pt α dC-C = 137.5 pm α = 64E E dC-C = 146 pm R=F α = 80E E dC-C = 153 pm C=C occupies one coordination site d10 ML3 C=C in ML3 plane d8 ML4 C=C perpendicular to plane. C R Ph3P α = 32.5E E R = CN R cf: C Ph3P β C Pt γ C C C 134 pm C 120 pm R = Ph β = 140E E γ = 39E E dPt-C = 206/201 pm dC-C = 132 pm R = CF3 β = 140E E γ = 30E E dPt-C = 202/203 pm dC-C = 125.5 pm C Ph3P C 154 pm R R The alkyne can be a 2e donor or 4e donor; 4e is common in 5th & 6th period TM. Alkyne Complexes - Reactions R Co2(CO)8 C + RC CH R (CO)3Co C Co(CO)3 (CO)3Co C (CO)3Co CH2 Co(CO)3 H HCl MeOH When one alkyne substituent is H, acid may cause a rearrangement. R Catalysis: 3 R C C R + 2 CO Ph3Cr(Thf)3 R R R R R Allyl Metal Complexes Ψ3 C C Available Metal Orbitals π* C Ψ2 C C px dxz πn C py Ψ1 C C dyz π C Ligand Molecular Orbitals s pz d z2 8 Allyl Metal Complexes 1. Allylic carbon atoms sp2 hybridized, pCCC .120E 2. dC-C ≅ 140 pm 3. Metal atom out of C3 plane, below centroid of the triangle formed by the three carbon atoms. 4. Terminal dM-C are commonly 5-15 pm longer than central dM-C. 5. 2-Allyl substituents commonly tilted toward metal atom - a result of pπ orbital tilting. 6. When a coordination plane can be identified the C3 plane forms a dihedral angle of from 95E to 120E with the coordination plane. Allyl Metal Complexes C O 139 pm 188 pm W 75EE 210 pm 20E E 3 198 pm 3 (η η -C3H5)Co(CO)3 Co C O 5 (η η -C5H5)(η η -C5H5)W(CO)2 C O C O C O 140 pm 206 pm 120E E Ni 198 pm CH3 202 pm H3C 12E E Br C O Fe 139 pm 213 pm C C O O 3 (η η -C3H5)Fe(CO)3Br Bis(2-methylallyl)Ni(0) Conjugated Polyenes & Cyclopolyenes 9 Aromatic and Non-aromatic Cyclopolyenes Non-aromatic Cyclopolyenes Huckel 4n + 2 Aromatic Cyclopolyenes n = 0 2e systems 2+ + n = 1 6e systems - 2- + 2+ n = 2 10e systems - 2- Sandwich Compounds an Isoelectronic 18e- Series 6 Cr Cr 6 6 5 6 7 6 Mn 5 Fe 7 5 5 Co 8 5 5 Ni 9 10 3 4 Dicyclopentadienyls of the First Period 5 V 5 5 Cr 5 Mn 6 5 5 5 5 Fe 7 5 Co 8 5 Ni 9 10 5 5 5 All dicyclopentadienyl complexes are Low Spin Complexes (Strong Field) except manganese which exhibits Hi/Low Spin temperature equilibrium. d3 d5 d4 d7 d6 d8 Dicyclopentadienyliron - Ferrocene Cyclopentadiene Molecular Orbitals Ferrocene Molecular Orbitals e2 e1 dxy a s dz2 a1g e2g e 2u nonbonding dxz pz a2u No suitable metal orbitals e1g dx2-y2 e1u e e2g 2u nonbonding dyz px e1g No suitable metal orbitals py e1u 10