* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Diagnostic and Therapeutic Cardiovascular Procedures
Cardiac contractility modulation wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Cardiovascular disease wikipedia , lookup
Heart failure wikipedia , lookup
Electrocardiography wikipedia , lookup
Saturated fat and cardiovascular disease wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Cardiac surgery wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Coronary artery disease wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
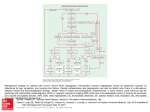
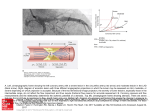
Diagnostic and Therapeutic Cardiovascular Procedures Right Heart Catheterization, Coronary Angiography, and Percutaneous Coronary Intervention Sripal Bangalore, MD, MHA; Deepak L. Bhatt, MD, MPH heparinized saline to ensure an air-free system; stopcocks should be placed on the end of the ports. The balloon inflation syringe should be filled with 1.5 mL of air and the balloon inflated under saline to ensure there are no air leaks in the balloon. The pressure monitoring system should be prepared for use according to institutional practice to ensure an air-free system. The PA catheter can be inserted either under fluoroscopic guidance (preferred) or under the guidance of the pressure waveforms. Fluoroscopic guidance is recommended in patients with a markedly enlarged right atrium or ventricle or severe tricuspid regurgitation or in those with left bundlebranch block. The catheter should be advanced to the vena cava/right atrium junction (10 –15 cm from the internal jugular vein or 25–30 cm from the femoral vein) and the balloon inflated. The catheter is designed to be flow-directed and will aid the flow of the catheter along the direction of blood flow (right atrium to PA). Pressure measurements and sampling of blood for measurement of oxygen saturation can be performed as the catheter is advanced through the various chambers of the heart, although it is sometimes easier to advance distally to the PA and make measurements on the way back out (that is, if the PA catheter is not meant to stay in place). The sequential changes in the pressure waveform are as shown in Figure 1. Once a pulmonary capillary wedge pressure tracing is seen, the balloon should be deflated and the catheter pulled back by 1 to 2 cm to remove any redundant length or loop in the right atrium or ventricle. The tip should be maintained in a position where full or near-full inflation volume is necessary to produce a wedge tracing. The ideal position of the catheter is the zone 3 region of the lung (lower zone). For subsequent wedge tracings, the balloon should be inflated with the minimum amount of air to produce a wedge tracing. Excess can cause overwedging, in which the pulmonary capillary wedge pressure will be higher because of transmittal of pressure from the balloon and with loss of characteristic waveforms. For precautions, please refer to the accompanying slide set in the online-only Data Supplement. Right-Sided Heart Catheterization Flow-directed pulmonary artery catheters, also called SwanGanz catheters after inventors Jeremy Swan and William Ganz, were initially used to guide therapy after acute myocardial infarction,1 but are now used in a variety of settings. There is, however, no universally accepted indication for their use, because right-sided heart (pulmonary artery [PA]) catheterization has not been shown to improve outcome.2 Downloaded from http://circ.ahajournals.org/ by guest on August 9, 2017 Indications and Contraindications PA catheterization is useful in a number of diagnostic applications. It has been used in the differentiation of various causes of shock and pulmonary edema; the evaluation of pulmonary hypertension; the differentiation of pericardial tamponade from constrictive pericarditis and restrictive cardiomyopathy; the diagnosis of left-to-right intracardiac shunts; and to guide fluid management and hemodynamic monitoring of patients after surgery or complicated myocardial infarction and for patients in shock, heart failure, etc. Although there is no absolute contraindication for use of PA catheters, care should be taken in patients with severe pulmonary hypertension and in the elderly. Fluoroscopic guidance is recommended in patients with preexisting left bundle-branch block, because it likely reduces the risk of right bundle-branch damage during catheter insertion and consequent complete heart block. PA (Swan-Ganz) Catheter: Insertion Technique The PA catheter can be inserted via the internal jugular vein, subclavian vein, antecubital vein, or femoral vein. After the site is prepped and draped, local anesthesia is administered to the site with 5 to 10 mL of 2% lidocaine by use of a 25-gauge needle. The vein is entered with a needle, preferably by micropuncture and preferably under ultrasound guidance (especially for the internal jugular vein), and an introducer catheter is inserted by use of a standard technique. Under sterile conditions, the PA catheter should be removed from the packaging and the proximal and distal ports flushed with From the New York University School of Medicine (S.B.), New York, NY, and the VA Boston Healthcare System, Brigham and Women’s Hospital, and Harvard Medical School Boston, MA (D.L.B.). The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA. 111.065219/-/DC1. Hyperlinked to this article is a video that shows the following 3 procedures: Right-sided heart catheterization, coronary angiography, and percutaneous coronary intervention. All 3 procedures were performed by Drs Sripal Bangalore and Deepak Bhatt at the VA Boston Healthcare System, West Roxbury Campus. Correspondence to Deepak L. Bhatt, MD, MPH, FAHA, Chief of Cardiology, VA Boston Healthcare System, Director, Integrated Interventional Cardiovascular Program, Brigham and Women’s Hospital and the VA Boston Healthcare System, Senior Investigator, TIMI Study Group, Associate Professor of Medicine, Harvard Medical School, 1400 VFW Parkway, Boston, MA 02132. E-mail [email protected] (Circulation. 2011;124:e428-e433.) © 2011 American Heart Association, Inc. Circulation is available at http://circ.ahajournals.org DOI: 10.1161/CIRCULATIONAHA.111.065219 e428 Bangalore and Bhatt Cardiac Catheterization and PCI e429 Figure 1. Diagram shows sequential pressure waveforms as the pulmonary artery catheter traverses through the various chambers of the heart. Pressure Recordings Downloaded from http://circ.ahajournals.org/ by guest on August 9, 2017 Pressure should always be recorded at end expiration (except in patients on positive end-expiratory pressure), as under normal conditions, pressures will be lower in inspiration because of the decrease in intrathoracic pressure. Before any pressure measurements are taken, it is imperative to perform zeroing and referencing of the system. Zeroing is accomplished by opening the system to air so as to equilibrate with atmospheric pressure and referencing by ensuring that the air-fluid interface of the transducer is at the level of the patient’s heart (phlebostatic axis; fourth intercostal space midway between anterior and posterior chest wall). It is imperative to ensure that the transducer is at the level of the heart, because for every inch the heart is offset from the reference point of the transducer, a 2-mm Hg degree of error will be introduced. If the heart is lower than the transducer, the pressure will be erroneously low, and if the heart is higher, the pressure will be erroneously high. The dynamic response of the pressure monitoring system should be determined by measuring the resonant frequency and the damping coefficient of the system by use of the fast-flush test, as described in Figure 2. Pressure wave interpretations and differential for the common wave patterns from the right atrial and pulmonary capillary wedge pressure tracings are shown in Table 1. Cardiac Output Measurement Three indirect methods for cardiac output determinations are (1) the dye indicator dilution technique, (2) the Fick technique, and (3) the thermodilution technique. The Fick and thermodilution techniques are the most widely used, and the former is considered the gold standard for cardiac output measurement. The Fick principle is based on the observation that the total uptake of (or release of) oxygen by the peripheral tissues is equal to the product of the blood flow to the peripheral tissues and the arterial-venous concentration difference (gradient) of oxygen. It is therefore based on measurement of the ratio of oxygen consumption to oxygen extraction. Oxygen consumption can be measured with an oxygen hood or estimated to be 250 mL/min or 125 mL/min per square meter of body surface area under resting conditions. Oxygen extraction is measured as the arteriovenous oxygen difference, which is given by the following formula: 13.4⫻hemoglobin concentration⫻(SaO2⫺SvO2). SaO2 is the arterial oxygen saturation, whereas SvO2 is the mixed venous oxygen saturation, measured as (3⫻superior vena cava saturation⫹inferior vena cava saturation)/4. This is most accurate in low-output states and is considered the “gold standard.” In the thermodilution technique, a known amount of solution (usually saline) is injected into the proximal port (right atrium), where it mixes and cools the blood, which is recorded by a thermistor located at the distal end of the catheter. Cardiac output is inversely proportional to the area under the curve. The thermodilution technique is not reliable in patients with severe tricuspid or pulmonic valve regurgitation, because it results in lower peak and a prolonged washout phase due to recirculation that results in underestimation of cardiac output. It is also not reliable in patients with intracardiac shunts (it overestimates cardiac output). Derived Parameters PA catheter measurements can also be used to calculate systemic and pulmonary vascular resistance, stroke work index, shunt fraction, and valve area (Table 2). However, the vascular resistance obtained is the least accurate (of the measures obtained from the catheter) and the most sensitive Figure 2. Fast-flush test/square-wave testing is performed by briefly opening and closing the valve in the continuousflush device, which produces a squareware pattern on the oscilloscope: an initial steep rise, followed by a plateau, followed by a steep fall below baseline, which is then followed by oscillations. The pattern determines optimal vs suboptimal damping. e430 Circulation Table 1. Pressure Wave Interpretation Wave Pattern Cannon ‘a’ wave Tall ‘a’ wave October 25, 2011 Mechanism Condition AV dissociation Complete heart block, ventricular tachycardia, AVNRT Increased atrial pressure Mitral or tricuspid stenosis Table 3. Hemodynamic Parameters That Help Differentiate Constrictive Pericarditis From Restrictive Cardiomyopathy Parameter LVEDP-RVEDP, mm Hg ⱕ50 ⬎50 ⬎1/3 ⱕ1/3 Atrial fibrillation Tall ‘v’ wave Increased volume during ventricular systole Mitral or tricuspid insufficiency, VSD RV/LV pressure waveform Loss of ‘y’ descent Equalization of diastolic pressures Cardiac tamponade RA pressure waveform Rapid diastolic filling Constrictive pericarditis Downloaded from http://circ.ahajournals.org/ by guest on August 9, 2017 to minor inaccuracies in data acquisition. Hemodynamic parameters that help differentiate constrictive pericarditis from restrictive cardiomyopathy are outlined in Table 3. Coronary Angiography Coronary artery disease is the leading cause of death for both men and women in the United States. More than 1.5 million cardiac catheterizations are performed every year in the United States, primarily to diagnose coronary artery disease. The indications for diagnostic coronary angiography are listed in the accompanying slide sets, and more detailed Table 2. Derived Parameters Using a Pulmonary Artery Catheter Parameter Formula Normal Values Systemic vascular resistance 共MAP–RAP兲⫻80 CO 700 to 1600 dyne 䡠 s⫺1 䡠 cm⫺2 (9 –20 Wood units) Pulmonary vascular resistance 共MPAP–PCWP兲⫻80 CO 20 to 120 dyne 䡠 s⫺1 䡠 cm⫺2 (0.25–1.5 Wood units) Stroke work index 共MPAP–LVEDP兲⫻SVI⫻ 0.0136 45 to 75 g 䡠 m⫺1 䡠 m⫺2 䡠 beat⫺1 (LV) 5 to 10 g 䡠 m⫺1 䡠 m⫺2 䡠 beat⫺1 (RV) Shunt fraction Mitral valve area (Gorlin’s equation) Aortic valve area (Gorlin’s equation) Aortic valve area (Hakki equation) 共SaO2⫺MvO2) (PvO2⫺PaO2) CO (ml/min) 37.7⫻DFP⫻HR⫻ 冑⌬P CO (ml/min) 44.3⫻SEP⫻HR⫻ 冑⌬P CO 共L/min兲 冑⌬P 1 4 to 6 cm2 3 to 4 cm2 3 to 4 cm2 MAP indicates mean arterial pressure; RAP, right atrial pressure; CO, cardiac output; MPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; LVEDP, left ventricular end-diastolic pressure; SVI, stroke volume index; LV, left ventricle; RV, right ventricle; SaO2, oxygen saturation, arterial; MvO2, oxygen saturation, mixed venous; PvO2, oxygen saturation, pulmonary veins; PaO2, oxygen saturation, pulmonary artery; DFP, diastolic filling period; HR, heart rate; SEP, systolic ejection period; and ⌬P, mean pressure gradient. Please note for the Hakki equation, peak-to-peak gradient can be used instead of the mean systolic gradient. ⬎5 RVEDP/RVSP, mm Hg Loss of atrial kick AV indicates atrioventricular; AVNRT, atrioventricular nodal reentry tachycardia; and VSD, ventricular septal defect. ⱕ5 Restrictive Cardiomyopathy RV systolic, mm Hg No ‘a’ wave Exaggerated ‘y’ descent Constrictive Pericarditis RV/LV interdependence PCWP/LV respiratory gradient Discordance Concordance Dip and plateau (square root sign) Dip and plateau (square root sign) Prominent ‘y’ descent Prominent ‘y’ descent ⱖ5 ⬍5 LVEDP indicates left ventricular end-diastolic pressure; RVEDP, right ventricular end-diastolic pressure; RV, right ventricular; RVSP, right ventricular systolic pressure; LV, left ventricular; RA, right atrial; and PCWP, pulmonary capillary wedge pressure. information can be obtained from the American College of Cardiology/American Heart Association guidelines.3 Although there are no absolute contraindications to cardiac catheterization, relative contraindications include coagulopathy, such as patients taking warfarin or after thrombolytic therapy (a radial approach can be attempted based on urgency); decompensated congestive heart failure; uncontrolled hypertension; pregnancy; inability of the patient to cooperate; active infection; renal failure; and contrast medium allergy. Coronary Angiography: Technique After a through history has been obtained, a thorough physical examination has been performed, and written informed consent has been obtained from the patient, conscious sedation with a narcotic and a benzodiazepine should be used before vascular access is attempted. Vascular access can be either femoral (described by Bangalore and Bhatt3a in the section on vascular access and closure devices), radial, or brachial. The selected diagnostic catheter should be flushed with saline to ensure an air-free system. Once arterial access is obtained (as described in the section on vascular access and closure devices), a catheter of appropriate size and configuration is advanced over a 0.035- or 0.038-inch guidewire. Once it is in the ascending aorta, the guidewire is removed, and the catheter is allowed to bleed back to remove any thrombus or atherosclerotic debris. The catheter is then connected to a manifold assembly connected to a pressure transducer for continuous central pressure monitoring and is flushed to ensure an air-free system. Before any pressure is measured, zeroing and referencing must be performed. The transducer should be opened to air to zero out the system. Care must be taken to ensure that the pressure transducer is at the level of the phlebostatic axis, which is roughly the midportion between the anterior and posterior chest wall along the left fourth intercostal space. The central aortic pressure should be recorded and compared with the cuff measured brachial pressure. If there is a considerable difference between the 2, subclavian artery stenosis should be in the differential. The catheter should then be filled with 3 to 4 mL of contrast and advanced to engage Bangalore and Bhatt the coronary ostium, in the left anterior oblique (LAO) projection. After ensuring that there is no ventricularization or damping of the pressure, 2 to 3 mL of contrast should be injected to confirm the position of the catheter in the coronary ostium. Coronary angiography should be performed in standard views in orthogonal planes to visualize the lesion and serve as a roadmap for percutaneous coronary intervention (PCI). Nonstandard views should be considered on the basis of the lesion location, orientation of the heart, and patient body habitus. Before contrast is injected, with every view, care should be taken to ensure there is no ventricularization or damping of the pressure waveforms. Complications Downloaded from http://circ.ahajournals.org/ by guest on August 9, 2017 The overall risk of major complications with coronary angiography is 1% to 2%. This includes death, myocardial infarction, stroke, bleeding, vascular complications, and contrast reactions. Catheter Selection Selecting the right catheter is important, and the choice depends on the following: 1. Access site: Choice of catheters depends to a certain degree on the access site (femoral versus radial versus brachial). 2. Aortic width: Normal aortic width is 3.5 to 4.0 mm, narrow is ⬍3.5 mm, and dilated is ⬎4.0 mm. 3. Coronary ostial location: High versus low; anterior versus posterior. 4. Coronary ostial orientation: Superior, inferior, horizontal, or shepherd’s crook (for right coronary artery only). It is preferable to use standard workhorse catheters for routine coronary angiography, such as the Judkins right size 4 (JR4) and Judkins left size 4 (JL4). It is important to always ensure coaxial alignment of the catheter. Catheters have 2 main curves: A primary (distal) curve and a secondary (proximal) curve. The distance between the 2 curves denotes the length of the catheter. As a general rule, shorter-curve catheters are better suited for superior ostial takeoffs, whereas the longercurve catheters are better suited for inferior ostial takeoffs. Standard Angiographic Views The standard angiographic views for the left and right coronary arteries are detailed in the slide set. For the left coronary artery, many operators follow a standard order of views, such as starting with the right anterior oblique (RAO) caudal (RAO 20°, caudal 20°), PA cranial (PA 0°, cranial 30°), shallow RAO cranial (RAO 10°, cranial 40°), LAO cranial (LAO 50°, cranial 30°), LAO caudal (LAO 50°, caudal 30°), and PA caudal (PA 0°, caudal 30°) for the left coronary system. For the right coronary artery, LAO 30°, RAO 30°, and PA cranial (PA 0°, cranial 30°) are frequently used. Nonstandard views are used if the standard views are not clear and if additional information about a lesion is needed. Cardiac Catheterization and PCI e431 Systematic Interpretation of Coronary Angiography A systematic interpretation of a coronary angiogram would involve evaluation of the extent and severity of coronary calcification and lesion quantification in at least 2 orthogonal views assessing for severity, calcification, presence of ulceration/thrombus, degree of tortuosity, American College of Cardiology/American Heart Association lesion classification, and reference vessel size. In addition, grading of the TIMI (Thrombolysis In Myocardial Infarction) flow and that of the TIMI myocardial perfusion blush grade should be performed. Also, it is important to identify and quantify the coronary collaterals. Some of the definitions are included in Table 4. Percutaneous Coronary Intervention It is estimated that more than 1.2 million PCI procedures are performed every year in the United States. The indications for PCI are listed in the accompanying slide sets, and more detailed information can be obtained from the American College of Cardiology/American Heart Association guidelines on percutaneous coronary intervention and the appropriateness criteria.7–9 The contraindications and cautions are similar to those described under coronary angiography. PCI Technique After the clinician has ensured that the patient understands the procedure and has provided informed consent, conscious sedation is administered with a narcotic and a benzodiazepine. As described under the section on coronary angiography, vascular access can be femoral, radial, or brachial. Antiplatelet therapy with aspirin should be given before the procedure, and a loading dose of thienopyridine (clopidogrel or prasugrel) should be given periprocedurally. Antithrombotic therapy with unfractionated heparin, low-molecular-weight heparin, or bivalirudin should be given and the activated clotting time maintained at 250 to 300 seconds (for unfractionated heparin given as monotherapy). Glycoprotein receptor IIb/IIIa inhibitors can also be used based on the procedure. The selected guiding catheter (connected to a Y-port) should be flushed with saline to ensure an air-free system. Once arterial access is obtained (as described by Bangalore and Bhatt3a in the section on vascular access and closure devices), a guiding catheter of appropriate size and configuration is advanced over a 0.035- or 0.038-inch guidewire. Once in the ascending aorta, the guidewire is removed, and the catheter is allowed to bleed back to remove any thrombus or atherosclerotic debris. The catheter is then connected to a manifold assembly that is connected to a pressure transducer for continuous central pressure monitoring. The guiding catheter is flushed to ensure an air-free system and should then be filled with 3 to 4 mL of contrast and advanced to engage the coronary ostium, in the LAO 30° projection. After ensuring that there is no ventricularization or damping of the pressure, 2 to 3 mL of contrast should be injected to confirm the position of the catheter in the coronary ostium. After adequate antithrombotic agents have been given, a 0.014-inch guidewire with a curve placed at its tip is advanced through the guide catheter into the coronary artery and across the lesion. At this stage, in the setting of thrombotic ST-elevation myocardial infarction lesions, a e432 Circulation October 25, 2011 Table 4. Standard Definitions for Interpretation of Coronary Angiography ACC/AHA lesion classification4 Type A lesion Minimally complex, discrete (length ⬍10 mm), concentric, readily accessible, nonangulated segment (⬍45°), smooth contour, little or no calcification, less than totally occlusive, not ostial in location, no major side branch involvement, and absence of thrombus Type B lesion Moderately complex, tubular (length 10 to 20 mm), eccentric, moderate tortuosity of proximal segment, moderately angulated segment (⬎45°, ⬍90°), irregular contour, moderate or heavy calcification, total occlusions ⬍3 mo old, ostial in location, bifurcation lesions requiring double guidewires, and some thrombus present Type C lesion Severely complex, diffuse (length ⬎2 cm), excessive tortuosity of proximal segment, extremely angulated segments ⬎90°, total occlusions ⬎3 mo old and/or bridging collaterals, inability to protect major side branches, and degenerated vein grafts with friable lesions Lesion length, mm Discrete lesion length ⬍10 Tubular lesion length 10–20 Diffuse lesion length ⱖ20 Lesion angulation Downloaded from http://circ.ahajournals.org/ by guest on August 9, 2017 Moderate Lesion angulation ⱖ5° Severe Lesion angulation ⱖ90° Calcification Moderate Severe Densities noted only with cardiac motion before contrast injection Radiopacities noted without cardiac motion before contrast injection TIMI flow grades5 0 Absence of any antegrade flow beyond a coronary occlusion 1 (Penetration without perfusion) Faint antegrade coronary flow beyond the occlusion, with incomplete filling of the distal coronary bed 2 (Partial reperfusion) Delayed or sluggish antegrade flow with complete filling of the distal territory 3 (Complete perfusion) Normal flow that fills the distal coronary bed completely TIMI myocardial perfusion grades6 0 Either minimal or no ground glass appearance (blush) of the myocardium in the distribution of the culprit artery 1 Dye slowly enters but fails to exit the microvasculature. Ground glass appearance (blush) of the myocardium in the distribution of the culprit lesion that fails to clear from the microvasculature, and dye staining present on the next injection (approximately 30 seconds between injections) 2 Delayed entry and exit of dye from the microvasculature. There is a ground glass appearance (blush) of the myocardium that is strongly persistent at the end of the washout phase (ie, dye is strongly persistent after 3 cardiac cycles of the washout phase and either does not diminish or only minimally diminishes in intensity during washout) 3 Normal entry and exit of dye from the microvasculature. There is a ground glass appearance (blush) of the myocardium that clears normally and is either gone or only mildly to moderately persistent at the end of the washout phase (ie, dye is gone or is mildly/moderately persistent after 3 cardiac cycles of the washout phase and noticeably diminishes in intensity during the washout phase), similar to that in an uninvolved artery ACC/AHA indicates American College of Cardiology/American Heart Association; TIMI, Thrombolysis In Myocardial Infarction. manual aspiration catheter can be used to aspirate thrombus. For saphenous vein graft interventions, an embolic protection device should be deployed before any angioplasty or stenting (if feasible, as described in the upcoming article and section by Bangalore and Bhatt10 on embolic protection devices). An appropriate-size compliant balloon may now be advanced over this guidewire to the region of the stenosis and the balloon inflated with a mixture of 50:50 heparinized saline and contrast to predilate the lesion. The balloon is then removed. Predilation should be avoided in saphenous vein graft grafts (if possible) to reduce the risk of distal embolization. Once adequate predilation is performed, a stent of suitable type, size, and length is taken and is flushed to ensure an air-free system. This is advanced over the guidewire across the lesion and deployed by inflating the balloon-mounted stent to appropriate pressures. The stent balloon is now removed, and coronary angiography is performed to ensure no complications and to assess for adequate stent expansion. Some laboratories believe in routine postdilation of all stents (except saphenous vein graft interventions) to ensure adequate strut expansion. If desired, this is accomplished by use of a noncompliant balloon of appropriate size and length. The balloon is removed and coronary angiography performed in 2 orthogonal views to assess the following: Stent expansion; the proximal and distal stent edges, to ensure no dissections or perforation; the distal wire site, to ensure no perforation; and the ostium, to ensure no dissection caused by the guiding catheter. The guidewire is then removed, and further angiography is performed to confirm adequate stent deployment and no complications, as described above. The guiding catheter should then be removed and intravenous antithrombotic Bangalore and Bhatt therapy stopped unless further continuation is required because of heavy thrombus burden. Coronary Guide Catheter Selection For routine stenting of noncomplex lesions, a coronary guide catheter of 4F to 6F size is generally recommended. Larger guide catheters (7F to 8F) are needed for more complex procedures (bifurcation stenting, rotational atherectomy, or to provide extra support for chronic total occlusions, tortuous, or calcified lesions). The choice of guide catheter (standard versus support versus extra support) depends on the complexity of the lesion, presence of chronic total occlusions, vessel tortuosity, and calcification, as well as on operator preference. Coronary Guidewire Selection Downloaded from http://circ.ahajournals.org/ by guest on August 9, 2017 The types of guidewires are listed in the accompanying slide set. Some general principles of guidewire selection will be described. It is preferable to always start with the least traumatic wire (workhorse wire) and to always advance the wire under fluoroscopic guidance, ensuring that the guidewire tip does not buckle (more prone to dissection) and that the wire tip is always free. If frequent premature ventricular contractions are noted on wire advancement, the wire tip might have perforated; the wire should be withdrawn slightly. If the wire becomes trapped and is difficult to retrieve (especially in calcified arteries) even with gentle traction, it is best to use a low-profile balloon or small-caliber catheter and advance until the hinge point and then withdraw both as a unit. For stenting over a bifurcation, if a wire is placed in the side branch to protect the branch vessel, the stent can be deployed at low pressures, with withdrawal of the wire in the branch, recrossing through the stent strut, and postdilation to higher pressures. If the wire tip breaks off and embolizes, it potentially can be retrieved with a snare, and if that fails, the embolized tip may be plastered against the wall by use of a stent. Complications Apart from the complications listed under diagnostic coronary angiography in the accompanying slide set, those related to PCI include abrupt closure, dissection, perforation, intramural hematoma, side-branch occlusion, distal embolization, ventricular arrhythmia, acute renal failure, radiation injury, myocardial infarction, stroke, emergent coronary artery bypass grafting, and death. Most of these complications can be minimized by appropriate patient selection and with the use of proper catheterization techniques, although they cannot be eliminated entirely. Therefore, great care must always be exercised in deciding to proceed with catheterization and PCI. Acknowledgments We would like to thank the nurses in the VA Boston Catheterization Laboratory, who helped with the filming of the cases: Diane Lapsley, RN, Scott Sandstrum RN, and Kimberly Shattuck, RN. Disclosures Dr Bhatt receives research grants (significant) from Astra Zeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, Sanofi-aventis, and Cardiac Catheterization and PCI e433 The Medicines Company. Dr Bangalore serves on the advisory board for Daiichi Sankyo. References 1. Swan HJ, Ganz W, Forrester J, Marcus H, Diamond G, Chonette D. Catheterization of the heart in man with use of a flow-directed balloontipped catheter. N Engl J Med. 1970;283:447– 451. 2. Sandham JD, Hull RD, Brant RF, Knox L, Pineo GF, Doig CJ, Laporta DP, Viner S, Passerini L, Devitt H, Kirby A, Jacka M. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348:5–14. 3. Scanlon PJ, Faxon DP, Audet AM, Carabello B, Dehmer GJ, Eagle KA, Legako RD, Leon DF, Murray JA, Nissen SE, Pepine CJ, Watson RM, Ritchie JL, Gibbons RJ, Cheitlin MD, Gardner TJ, Garson A Jr, Russell RO Jr, Ryan TJ, Smith SC Jr. ACC/AHA guidelines for coronary angiography: executive summary and recommendations: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Coronary Angiography) developed in collaboration with the Society for Cardiac Angiography and Interventions. Circulation. 1999;99:2345–2357. 3a. Bangalore S, Bhatt DL. Femoral arterial access and closure. Circulation. 2011;124:e147– e156. 4. Ryan TJ, Faxon DP, Gunnar RM, Kennedy JW, King SB 3rd, Loop FD, Peterson KL, Reeves TJ, Williams DO, Winters WL Jr, et al. Guidelines for percutaneous transluminal coronary angioplasty: a report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Percutaneous Transluminal Coronary Angioplasty). Circulation. 1988;78:486 –502. 5. Gibson CM, Ryan KA, Kelley M, Rizzo MJ, Mesley R, Murphy S, Swanson J, Marble SJ, Dodge JT, Giugliano RP, Cannon CP, Antman EM; TIMI Study Group. Methodologic drift in the assessment of TIMI grade 3 flow and its implications with respect to the reporting of angiographic trial results. Am Heart J. 1999;137:1179 –1184. 6. Gibson CM, Cannon CP, Murphy SA, Ryan KA, Mesley R, Marble SJ, McCabe CH, Van De Werf F, Braunwald E. Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs. Circulation. 2000;101:125–130. 7. Kushner FG, Hand M, Smith SC Jr, King SB 3rd, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE Jr, Green LA, Hochman JS, Jacobs AK, Krumholz HM, Morrison DA, Ornato JP, Pearle DL, Peterson ED, Sloan MA, Whitlow PL, Williams DO. 2009 Focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update): a report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205–2241. 8. Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/ SCAI/STS/AATS/AHA/ASNC 2009 appropriateness criteria for coronary revascularization: a report by the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology: endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2009;53:530 –553. 9. Smith SC Jr, Feldman TE, Hirshfeld JW Jr, Jacobs AK, Kern MJ, King SB 3rd, Morrison DA, O’Neil WW, Schaff HV, Whitlow PL, Williams DO, Antman EM, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines (ACC/ AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention). Circulation. 2006;113:e166 – e286. 10. Bangalore S, Bhatt DL. Embolic protection devices. Circulation. In press. Right Heart Catheterization, Coronary Angiography, and Percutaneous Coronary Intervention Sripal Bangalore and Deepak L. Bhatt Downloaded from http://circ.ahajournals.org/ by guest on August 9, 2017 Circulation. 2011;124:e428-e433 doi: 10.1161/CIRCULATIONAHA.111.065219 Circulation is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 2011 American Heart Association, Inc. All rights reserved. Print ISSN: 0009-7322. Online ISSN: 1524-4539 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://circ.ahajournals.org/content/124/17/e428 Data Supplement (unedited) at: http://circ.ahajournals.org/content/suppl/2011/10/24/124.17.e428.DC1 Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Circulation can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Circulation is online at: http://circ.ahajournals.org//subscriptions/