* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Detecting Proteins that Interact with the Mbp1 Protein Using Yeast
Survey
Document related concepts
Transcript
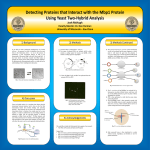
Detecting Proteins that Interact with the Mbp1 Protein Using Yeast Two-Hybrid Analysis Josh McHugh Faculty Mentor: Dr. Dan Herman University of Wisconsin – Eau Claire 1) Background 2) Methods 3) Methods Continued In an effort to better understand morphogenesis in Candida albicans – the process by which it transforms from the yeast form to the filamentous form – we are in the process of performing a yeast two-hybrid analysis to look at the interactions of the protein Mbp1 with itself, Swi6, and Skn7. It has been shown in previous research that Mbp1 plays an integral role in this process under nitrogen limiting conditions. By performing the aforementioned analysis, we hope add to the understanding of how morphogenesis takes place. 1) Genomic DNA is isolated from C. albicans 2) Two types of plasmid DNA are isolated: pGAD, which contains a coding region for leucine, and pGBK, which contains a coding region for tryptophan 4) The yeast Saccharomyces cerevisiae is then transformed to take in the plasmid and the gene fragment, at which point it will insert the gene into the plasmid. S. cerevisiae mating type a will be transformed with pGAD and Mbp1, while mating type α will be transformed with pGBK and one of Mbp1, Swi6, or Skn7. pGADMbp1 S. cerevisiae type a Fig. 2: pGAD and pGBK plasmid vectors. 3) Genes for Mbp1, Swi6, and Skn7 are replicated from the genomic DNA via PCR pGBKMbp1 pGBKSwi6 S. cerevisiae type α Fig. 1: Yeast and filamentous morphologies of C. albicans. pGBKSkn7 Fig. 4: Transformation of S. cerevisiae types a and α. 4) Discussion Yeast two-hybrid analysis is a procedure that detects physical interactions between proteins, and it relies on the fact that transcription factors have distinct binding and activating domains. These domains do not need to be connected for the transcription factor to function; they simply need to be close together. In order to perform this test, the binding domain is attached to Mbp1 and the activating domain is attached separately to Mbp1, Swi6, and Skn7. If Mbp1 interacts with any of these proteins, the two domains will be brought together, and transcription of the reporter gene histidine will ensue. This will allow the yeast to survive on an agar plate lacking histidine. Fig 3: Gel Electrophoresis results of genes replicated from genomic DNA. Lanes from left to right: ladder, Mbp1, Mbp1, Swi6, Skn7, ladder. Streak 1: S. cerevisiae type a Streak 2: S. cerevisiae type α 5) Acknowledgements We would like to thank the following for their support of our research: • The Office of Research and Sponsored Programs Fig 6: Diagram of yeast two-hybrid procedure. 5) The S. cerevisiae with pGADMbp1 are selected for by being grown on plates without leucine, and those with any of the pGBK plasmids are grown on plates without tryptophan for selection 6) PCR is then performed to verify that the yeast strains contain the plasmid 7) The pGADMbp1 strain is then separately crossed with each of the pGBK strains in order to produce the diploid form of S. cerevisiae, which would be able to survive on a plate lacking both leucine and tryptophan. Images from: • http://www.kent.ac.uk/bio/muhlschlegel/research.html • http://www.clontech.com/images/pt/PT3249-5.pdf • http://www.clontech.com/images/pt/PT3248-5.pdf • http://www.clontech.com/images/1024.gif Location of diploid S. cerevisiae. Fig. 5: S. cerevisiae mating cross technique. 8) Each successful cross is then transferred to a plate without leucine, tryptophan, and histidine to test for protein interaction.





![NUTRICELL START [en tête: NUTRIENTS]](http://s1.studyres.com/store/data/007854045_2-c4164e6cb36cf3b1ce13f2bee9ca3ea2-150x150.png)