* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download C - Aptagen
Nerve guidance conduit wikipedia , lookup
Stem-cell niche wikipedia , lookup
Cancer stem cell wikipedia , lookup
Embryonic stem cell wikipedia , lookup
Drug discovery wikipedia , lookup
Cellular differentiation wikipedia , lookup
Artificial cell wikipedia , lookup
Stem-cell therapy wikipedia , lookup
Induced pluripotent stem cell wikipedia , lookup
Hematopoietic stem cell wikipedia , lookup
Cell encapsulation wikipedia , lookup
“Forget Antibodies. Use Aptamers!”
Presentation Contents:
1. Introduction and Background
2. Aptamer Introduction
3. Diagnostic Applications
4. Drug Discovery Applications
Examples of Aptamer Shapes
A.
B.
C.
D.
A.
B.
C.
D.
Pseudoknot (ligand for HIV-1 reverse transcriptase)
G-quartet (ligand for thrombin)
Hairpin (ligand for bacteriophage for T4 polymerase)
Stem loop/bulge (ligand for ATP)
taken from McGown, et.al. (1995)
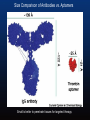
Size Comparison of Antibodies vs. Aptamers
Small is better to penetrate tissues for targeted therapy.
Biosensor and Biochip Platforms
Improved
signal closer to
surface.
Aptamers easily
tethered to solid
interface through a
wide variety of
conjugation
chemistries.
Detection of binding event intact
- pM to nM affinity
- Engineer out cross-reactivity…eliminate
false positives
(10,000 fold specificity, e.g. Theophylline/Caffeine)
- Ligand binding against unknown and
undiscovered biomarkers
- Manufacturing (pennies on the dollar)
- Stability (long shelf-life; heat denature/refold)
Apta-index™
(database of aptamers)
Whitepaper on Website
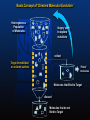
Basic Concept of ‘Directed Molecular Evolution’
Heterogeneous
Population
of Molecules
’sloppy’ copy
to explore
mutations
collect
Target immobilized
on column surface
‘Fittest’
molecules
Molecules that Bind to Target
discard
Molecules that do not
Bind to Target
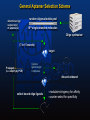
General Aptamer Selection Scheme
determine oligo
sequence(s)
of aptamer(s)
random oligonucleotide pool
1014 single-stranded molecules
Oligo synthesizer
(7 to 15 rounds)
target
Propagate
(i.e. amplify by PCR)
Capture
ligand-target
complexes
discard unbound
collect bound oligo ligands
- modulate stringency for affinity
- counter-select for specificity
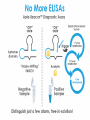
Diagnostic Applications
Traditional Antibody-based Diagnostic Method
(“Sandwich” OR ELISA Assays)
Plate coated with capture antibody
Add samples
Add detection antibody
Add substrate
Incubation steps and wash steps before detection = total time >>2 hours
Apta-beacon™ (GQ-EXPAR)
Demonstration Kit (Theophylline/Caffeine)
Caffeine
Theophylline
Selectivity:
Distinguish just a few Atoms!
Reconfigure
Contact Us for DEMO Kit
(Current Platform)
Colorimetric
Test Strip
(Lateral Flow Platform)
Sample Type:
Blood
Urine
Saliva
Environmental
etc…
Test Strip (Y/N)
Short-term Vision
Colorimetric Detection
Fluorescent Detection
Long-term Vision
Quantify
….NO Capturing. NO Washing. Just READ™….
Drug Discovery Applications
Pharmaceutical Drug Development Process
Success
Rate
5
Enter human clinical trials
> 8 years
>$1B
Animal Testing
of Drug candidates
5000
In vitro or in vivo assays
on drug candidates
Knowledge of Target / Mechanism
Pharmaceutical Drug Development
(combinatorial, natural product screening, etc.)
MASS SCREENING
Drug Discovery Process
(time consuming and labor intensive)
Random
High Volume
Screening
In Vitro
Studies
In Vivo
Studies
Clinical Studies
Humans
Combinatorial
Chemistry
A positive hit in a “test” tube environment does not
necessarily translate into a success in an in vivo
environment. Compound has to be re-engineered and
tested again in test tube, then back to animal. Back and
forward through this iterative process costs time
and money.
http://images.google.com/images?q=drug+discovery&btnG=Search&hl=en&lr=&ie=UTF-8
Aptagen’s Drug Discovery in Whole-Animal Models
(Saving Time and Money)
X
X
Random
High Volume
Screening
In Vitro
Studies
In Vivo
Studies
Clinical Studies
Humans
Combinatorial
Chemistry
By eliminating the “test” tube step, and
performing drug discovery ‘directly’ in an animal
model, we are one step closer to human clinical
trials, thereby saving time and money.
http://images.google.com/images?q=drug+discovery&btnG=Search&hl=en&lr=&ie=UTF-8
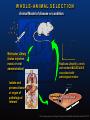
WHOLE-ANIMAL SELECTION
Animal Model of disease or condition
Molecular Library
(bolus injection,
nasal, or oral
administration)
Isolate and
process tissue
or organ of
pathological
interest
Replicate (Amplify), enrich,
and reselect MOLECULES
associated with
pathological marker
Pathological
Marker
Normal Tissue
Area
http://images.google.com/images?q=drug+discovery&btnG=Search&hl=en&lr=&ie=UTF-8
In drug development, DELIVERY is always an issue!
off-target effects…
Selection in Whole-Animals solves DELIVERY issues.
(Use molecular bullet to attach a known drug to increase specificity)
vehicle to carry cargo= toxin OR rescue failed drug candidates.
Chemical Diversity solves drug-like effects.
Potential for ‘smart’ molecular bullets
with Drug-like properties
Initial round
Progression of
Selection
with gradual
disappearance
of pathological
marker…
Normal tissue no sign of
pathology
Nth round
of ‘natural’
selection…
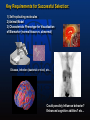
Key Requirements for Successful Selection:
1) Self-replicating molecules
2) Animal Model
3) Characteristic Phenotype for Visualization
of Biomarker (normal tissue vs. abnormal)
Disease, Infection (bacterial or viral), etc...
Could possibly Influence behavior?
Enhanced cognitive abilities? etc…
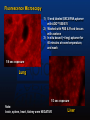
Fluorescence Microscopy
1) 5’-end labeled G9C4 RNA aptamer
with ADO™550/570
2) Washed with PBS & Fixed tissues
with acetone
3) In situ bound (~4 mg) aptamer for
40 minutes at room temperature,
and wash
1/6 sec exposure
Lung
1/3 sec exposure
Note:
brain, spleen, heart, kidney were NEGATIVE
Liver
Microscopy of Internalized Polyclonal Aptamer Library
(-) counter cells expressing mutant receptor
Figure 2B. Phase contrast and fluorescent images of (-) Mutant receptor cell line following
exposure to the TAMRA labeled G12 library. Mutant receptor cells, grown to 100% confluency
in a 100 mm TPP tissue culture dish, were exposed to 0.06 µM TAMRA labeled G12 library in
3ml of binding buffer (0.1mg/ml yeast tRNA, 1mg/ml BSA in wash buffer) for 30 minutes at
370C. The unbound library was aspirated from the dish (transferred to Positive target cells);
cells were washed twice with 5 mL wash buffer, scraped from their plate into 1 mL of wash
buffer. A 20 ul aliquot was placed on a glass slide for microscopy. Both the phase contrast
(left image) and fluorescent (right image) images were taken at 40X magnification of the same
field using a Tsview 1.4 MP CCD COOLED camera. These images suggest the library does not
bind to the (-) Mutant receptor cell line. [Ref: Notebook, NSR 3 – 43]
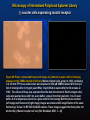
Microscopy of Internalized Polyclonal Aptamer Library
(+) target receptor expressing cells
Figure 2A. Phase contrast and fluorescent images of Target receptor cell line following
exposure to TAMRA labeled G12 library. Target cells, grown to 100% confluency in a 60mm
TPP tissue culture dish, were exposed to TAMRA labeled G12 library (3 ml of the unbound
fraction after (-) Mutant selection), for 30 minutes at 370C. The excess library was aspirated
from the dish; cells were washed twice with 5 mL wash buffer (1X PBS supplemented with 4.5
mg/mL glucose and 5mM MgCl2); scraped from their plate into 1 mL of wash buffer. A 20 ul
aliquot was placed on a glass slide for microscopy. Both the phase contrast (left image) and
fluorescent (right image) images were taken at 40X magnification of the same field using a
Tsview 1.4 MP CCD COOLED camera. The images suggest that the G12 library was
internalized. [Ref: Notebook, NSR 3 – 43]
Aptagen’s Capability Against a Wide Range of Targets
NextGEN Aptamers
(Aptabodies™)
It’s not an antibody. It’s not an aptamer.
It’s an “aptabody.”
Conceptual Relationships
aptabodyTM
aptamer
Naked nucleic acid
Conjugated nucleic acid
Functionalized
nucleic acid
Effective Drug
Delivery
Improve PK/PD
1-717- Aptagen
Aptagen, LLC
Jacobus/York, PA
AptabodyTM Library
(>1014 molecules)
unique SupraMolecular structures
(activity arises from the precise positioning
of functional groups within scaffold)
Diversity of Functional Groups
• organics
• metals
* fatty acids * amino acids
* sugars
* small molecule drugs
*molecular sizes are not relatively proportional
Comparison of Pharmaceutical Drug Formats
organics &
natural products
Biologics
Peptides & Proteins
Aptabody™
(postulated)
Nucleic Acid
Aptamer
Chemical Diversity
Large
Moderate
Small
Largest
Serum stability
Yes
Yes
Yes
Yes
DELIVERY
Moderate
Moderate
n/a
Yes
Drugs on the Market
Largest
Small
One
None
No
Yes
Yes
Yes
Moderate
Largest
Large (<30 KD)
Large (<60 KD)
‘In Vivo Selection’
Capability
Flexibility to Improve
PK/PD properties
Small (300-500D)
Moderate
Moderate to Largest (up to
180 KD for Antibody)
3’
Molecular Size
Smallest
C
pro
tyr
Most favorable
condition
C
A
ser
leu
T
Small molecule
drugs
N
val
5’
G
Apta-beacon™ Diagnostic Assay
(simple 1-step reaction, free in-solution)
Positive
Sample
Negative
Sample
Analyte
total time << 1 minute
….NO Capturing. NO Washing. Just READ….
Apta-switch™
(aptamer that produces a self-cleavage output signal)
Target
Metal Ions
Small Organics
Example
Co2+, Ni2+, Cd2+
Zn2+, Mn2+
Caffeine
Peptides
Rev Peptide
Proteins
Phosphorylated ERK2,
Unphosphorylted ERK2
FlashGel™
analysis
(5 minute run)
Apta-beacon Mechanism of Action
Aptamers that produce an immediate output signal for detection of target analyte.
Target
(e.g. metal ions, small molecules, proteins)
Q
F
Q
F
total time << 1 minute
Q strand
release
3’
5’
F
auto self-cleavage
Q
F
*
….NO Capturing. NO Washing. Just READ….
Aptagen’s Proprietary Signal Amplification System
(GQ-EXPAR)
strand
3’
release
initiate
5’
1st level
amplification
2nd level
amplification
BENEFITS:
Colorimetric Assay with Low Limits of Detection
Rapid Point-of-Use diagnostic against difficult
targets, e.g. Drugs of Abuse, Ebola, etc..
Color
Development
Delivery Applications
Preliminary Experiment: Targeting Major Organs & In Vivo Stability
Tail vein injection
2’-F-RNA library
(-) Library
nanomolar amounts
40 minutes
post-IV
Isolate various
organs/tissue
Tissue Harvesting
Purification of Rare 2’-F-RNA species
RT-PCR
Lane:
1
DNA
Ladder
2
3
no band
gel
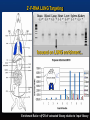
2’-F-RNA Targeting to Major Organs of the Mammalian Anatomy
2’-F-RNA LUNG Targeting
focused on LUNG enrichment...
Enrichment Ratio = qPCR of ‘extracted’ library relative to ‘input’ library
Enrichment RATIO
6.00E-02
5.00E-02
4.00E-02
3.00E-02
*
*
2.00E-02
*
1.00E-02
0.00E+00
G9
G9A2
LIBRARY
G9B1
G9C4
CLONES
Secondary
Structures
(MFOLD)
ΔG -43.49 kcal.mole-1
Tm 73.6oC
Family
# of Clones
Group A
12
Group B
Group C
ΔG -36.17 kcal.mole-1
Tm 75.6oC
ΔG -37.45 kcal.mole-1
Tm 65.9oC
5’- gggcgacccugaugag [Consensus Sequence] cgaaacggugaaagccguagguugccc -3’
[UGACUGCUCCGUUCCGUUAUGACAGCUGCACCCAGUUAAAGC:GGUUCUGGGUCCGGA]
G9A2
7
[CCUUUUUGAACAACUGUGCGAUUUGAUUG:AAAAUUCUCUCUGAUCCCACCGUGACG]
2
[UCUAGAGCGCAGAAACUUCUCUCAACGAUUCCCCACGUCCUCGCCCCGCCCGGU]
G9B1
G9C4
Aptamer Selection for Surface Binders
Template
LCR
Negative
Selection
G6-Gx
Circular DNA
PCR
Amplification of
bound aptamers
PC3
Positive
Selection
(G0-G5)
(Optional) PCR
Amplification of
unbound aptamers
PC3-PSMA
Unbound
aptamers
Bound
aptamers
WASTE
Figure 1. Schematic of Strategy. Linear template will undergo circularization via
LCR (Ligation Chain Reaction). The circularized aptamers will be incubated with PC3-PSMA
cells for positive selection. Aptamers specific for PSMA will be amplified; the selection
process will be repeated for approximately five generations, before beginning a negative
selection process with parental PC3 cells.
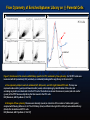
Flow Cytometry of Enriched Aptamer Library on (-) Parental Cells
A.
B.
PC3 Cells
G0
G19
unlabeled
Figure 7. Enrichment of the circular ssDNA library specific for PC3 monitored by flow cytometry. 5 x
105 PC3 cells were incubated with G0 (scrambled), G19 (enriched), or unlabeled (binding buffer only)
Figure 7. Enrichment of the circular ssDNA library specific for PC3 monitored by flow cytometry. 5 x 105 PC3 cells were
library for
30 min
at 4°C.
incubated
with
G0 (scrambled),
G19 (enriched), or unlabeled (binding buffer only) library for 30 min at 4°C.
a. Flow cytometry dotplot results of unlabeled (left), G0 (center), and G19 (right) labeled PC3 cells.
a.
dotplot
results
of (y-axis)
unlabeled
(left),
G0 (center),
G19 cell
(right)
labeled PC3
The top row
TheFlow
top cytometry
row represents
side
scatter
and
forward
scatterand
(x-axis)
morphology
bycells.
identification
represents side scatter (y-axis) and forward scatter (x-axis) cell morphology by identification of the cells, and
of the cells, and excluding any debris and dead cells from the PC3 cells. The bottom row shows
excluding any debris and dead cells from the PC3 cells. The bottom row shows fluorescence (x-axis) and side scatter
fluorescence
(x-axis)
and side scatter (y-axis)
the FITC
fluorescently-library
that has bound to the PC3
(y-axis)
of the FITC
fluorescently-library
that hasofbound
to the
PC3 cells.
cells.
Ref:[Notebook,
AN Priya Book 3, 124-127]
Ref:[Notebook, AN Priya Book 3, 124-127]
b. Histogram of flow cytometry Fluorescence intensity (x-axis) as a function of the number of viable cells (y-axis)
b. Histogram of flow cytometry Fluorescence intensity (x-axis) as a function of the number of viable
analyzed with Flowing Software v1.6.0. The G19 library (blue) is shifted to the right of the G0 (red) and unlabeled library
cells (y-axis)
analyzedwith
withPC3
Flowing
(black)
after incubation
cells. Software v1.6.0. The G19 library (blue) is shifted to the right of the
G0 (red) and unlabeled
library
after incubation with PC3 cells.
Ref:{Notebook,
AN Priya Book
3, (black)
124-127]
Ref:{Notebook, AN Priya Book 3, 124-127]
Flow Cytometry of Enriched Aptamer Library on (+) Cells
A.
B.
PSMA-PC3 Cells
unlabeled
G0
G19
Figure 6. Enrichment of the circular ssDNA library specific for PSMA-PC3 monitored by flow
cytometry. 2.5 x 105 PSMA-PC3 cells were incubated with G0 (scrambled), G19 (enriched), or unlabeled
Figure 6. buffer
Enrichment
of the circular
ssDNA
library specific for PSMA-PC3 monitored by flow cytometry. 2.5 x 105 PSMA-PC3
(binding
only) library
for 30 min
at 4°C.
cells were incubated with G0 (scrambled), G19 (enriched), or unlabeled (binding buffer only) library for 30 min at 4°C.
a. Flow cytometry dotplot results of unlabeled (left), G0 (center), and G19 (right) labeled PSMA-PC3
cells.
The cytometry
top row represents
side scatter
(y-axis)(left),
and G0
forward
scatter
celllabeled
morphology
by cells. The top row
a. Flow
dotplot results
of unlabeled
(center),
and (x-axis)
G19 (right)
PSMA-PC3
represents side
(y-axis)
and forward
cell
morphology
by identification
thebottom
cells, and excluding any
identification
of scatter
the cells,
and excluding
anyscatter
debris (x-axis)
and dead
cells
from the PSMA-PC3
cells.ofThe
debris
and fluorescence
dead cells from
the PSMA-PC3
cells. The
bottom
rowFITC-labeled
shows fluorescence
(x-axis)
and side
(y-axis) of the
row
shows
(x-axis)
and side scatter
(y-axis)
of the
library that
has bound
toscatter
the
FITC-labeled library that has bound to the PSMA-PC3 cells.
PSMA-PC3 cells.
Ref:{Notebook, AN Priya Book 3, 124-127]
Ref:{Notebook, AN Priya Book 3, 124-127]
b.
offlow
flowcytometry
cytometryFluorescence
Fluorescenceintensity
intensity(x-axis)
(x-axis)asasa afunction
functionofofthe
thenumber
number
viable
b. Histogram
Histogram of
ofof
viable
cells (y-axis) analyzed
with
Flowing
Software
v1.6.0.
The
G19
library
(blue)
is
shifted
to
the
right
of
the
G0
(red)
and
unlabeled
cells (y-axis) analyzed with Flowing Software v1.6.0. The G19 library (blue) is shifted to the right of thelibrary (black) after
incubation
with
PSMA-PC3
cells.
G0
(red) and
unlabeled
library
(black) after incubation with PSMA-PC3 cells.
Ref:{Notebook, AN Priya Book 3, 124-127]
Ref:{Notebook, AN Priya Book 3, 124-127]
Cell-based Selection for Intracellular-targeting Aptamers
intracellular target
Capture
ligand-target
complexes
Circular-ssDNA library
discard unbound
isolate intracellular bound oligo ligands
>100-fold preference for cells expressing intracellular target versus control cells