* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The REGULATE-PCI Randomized Clinical Trial
Survey
Document related concepts
Transcript
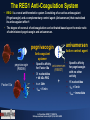
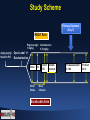
Effect of REG1 Anticoagulation System versus Bivalirudin on Cardiovascular Outcomes Following PCI: The REGULATE-PCI Randomized Clinical Trial Roxana Mehran, John Alexander, and Michael Lincoff on the Behalf of the REGULATE-PCI Investigators Conflicts of Interest Consulting: • AstraZeneca; Bayer; CSL Behring; Janssen Pharmaceuticals, Inc.; Merck & Co., Inc.; Osprey Medical Inc.; Regado Biosciences, Inc.; The Medicines Company; Watermark Consulting Scientific Advisory Board: • Abbott Laboratories; AstraZeneca; Boston Scientific Corporation; Covidien; Janssen Pharmaceuticals, Inc.; Merck & Co., Inc.; The Medicines Company; sanofi-aventis • Please visit websites https://www.mountsinai.org, https://www.dcri.org, hppts://www.my.clevelandclinic.org for comprehensive disclosures for the institutions and investigators Trial Organization Operations Academic Leadership Executive Committee •John Alexander (co-PI) •Michael Lincoff (co-PI) •Roxana Mehran (co-PI) •Paul Armstrong •Gabriel Steg •Christoph Bode •Steve Zelenkofske (Regado) Steering Committee: A. Levinson (USA), R. Becker (USA), V. Hasselblad (USA), K. Huber (Austria, P.R. Sinnaeve (Belgium), M. Aschermann (Czech Republic), P. Laanmets (Estonia), B. Merkely (Hungary), V. Guetta (Israel), M. Valgimigli (Italy), J.H. Cornel (Netherlands), J.D. Kasprzak (Poland), J. Morais (Portugal), B. Alekyan (Russia), V. Fridrich (Slovakia), J. Lopez/Sendon (Spain), R. Stables (UK), M. G. Cohen (USA), T. Povsic (USA) Project Management: DCRI, C5R, Regado, PAREXEL US Site Management: DCRI, C5R CN Site Management: CVC ROW Site Management: PAREXEL Data Management: DCRI Statistics: DCRI Safety: DCRI Clinical Event Committee: DCRI IXRS: ClinPhone Perceptive Informatics) Study Drug: Catalent / PAREXEL DSMB: Stanford U. – Robert Harrington (chair) BACKGROUND • Refinements in antithrombotic therapies have considerably enhanced the efficacy and safety of percutaneous coronary intervention (PCI), although no optimal strategy yet exists. • Platelet glycoprotein IIb/IIIa receptor antagonists reduce ischemic complications,1 but are accompanied by increased bleeding with associated mortality, morbidity and medical resource cost.2 • Bivalirudin reduces the risk of bleeding compared to heparin and glycoprotein IIb/IIIa inhibition, but is associated with higher rates of stent thrombosis and trends toward more frequent periprocedural myocardial infarction.3 What would be an ideal antithrombotic Regimen for PCI? • • • • Rapid Onset of Action Predictable Dose-Response High Anti-Thrombotic Efficacy Quick Reversibility or Titratability 1-Journal of the American College of Cardiology 2011;57:1190-9 2-New England Journal of Medicine 2009;360:2176-90 3-American Heart Journal 2008;155:369-74 The REG1 Anti-Coagulation System • REG-1 is a novel antithrombotic system Consisting of an active anticoagulant (Pegnivacogin) and a complementary control agent (Anivamersen) that neutralized its anticoagulant effect4. • The degree of reversal of anticoagulation can be titrated based upon the molar ratio of administered pegnivacogin and anivamersen. anivamersen pegnivacogin Active control agent Anticoagulant aptamer pegnivacogin (RB006) Factor IXa Specific affinity Specific affinity anivamersen for pegnivacogin for Factor IXa (RB007) with no other 31 nucleotides activity + 40 kDa PEG 15 nucleotides t1/2 > 24hr t1/2 < 5 min tmax < 5 min tmax ~ immediate + 4-Circulation 2008;117:2865-74. 5-European Heart Journal (2013) 34, 2481–2489 REG1 In the RADAR Trial • The phase 2, randomized, active-controlled RADAR trial showed that with a least 50% reversal of pegnivacogin by anivamersen, early vascular sheath removal was feasible and bleeding rates similar to heparin. • The composite of 30-day death, non-fatal MI, urgent target vessel revascularization, or recurrent ischemia in the target vessel was numerically lower in patients assigned to REG1 than Heparin (OR: 0.5; 95% CI: 0.2 – 1.4; p = 0.1). The majority of ischemic events were non-fatal periprocedural MIs. • In the RADAR study, 3 patients had allergic-like reactions shortly after pegnivacogin administration, of which 2 of these reactions were serious. 5-European Heart Journal (2013) 34, 2481–2489 The REGULATE-PCI Randomized Clinical Trial • Randomized, open-label, active-controlled, superiority, phase 3 trial to test the hypothesis that near complete FIXa inhibition with Pegnivacogin during PCI would provide a greater reduction in ischemic events than bivalirudin without increased bleeding as a result of anticoagulant reversal with Anivamersen. Study Scheme Primary Outcome (Day 3) REG1 Arm Pegnivacogin 1 mg/kg Angiography/ Need for PCI Anivamersen 0.5 mg/kg Open-Label 1:1 Randomization Dose PCI Bival Bolus End of Sheath PCI removal Bival Infusion Bivalirudin Arm FU Assessment 4-10d FU Visit 30 d Inclusion Criteria • Patients with CAD undergoing PCI stratified by 3 key subgroups: • Subgroup A: Patients with MI within prior 7 days - ischemic symptoms at rest and positive cardiac biomarkers • Subgroup B: Patients with at least one of the following risk factors: ACS with positive cardiac biomarkers > 7 days prior to randomization; unstable angina (without positive cardiac biomarkers); age > 70 years; diabetes; chronic kidney disease (estimated CrCl < 60 mL/min); planned multivessel PCI; prior CABG surgery; peripheral vascular disease; • Subgroup C: Patients with negative cardiac biomarkers and no risk factor, thereby not meeting criteria for Subgroup A or B. • Enrollment began with approximately 1000 patients from Subgroups B and C, with expansion to include the Subgroup A only after the safety of REG1 in lower-risk patients had been established. Pre-Procedural Treatment Investigators had to specify prior to randomization: • Procedural access (femoral or radial). • Vascular Closure Device use (yes/no) • Planned target vessel(s) • ADP/P2Y12 inhibitor (clopidogrel, prasugrel, or ticagrelor). Study Drug Administration • Pegnivacogin, bolus injection of 1.0 mg/kg over 2 minutes IV or via arterial sheath just prior to PCI procedure • Anivamersen, 0.5 mg/kg (80% reversal) IV over 1 minute upon completion of PCI. • REG1 Arm Second complete reversal dose of anivamersen (i.e. 0.5 mg/kg) to achieve 100% reversal could be administered at any time post PCI for bleeding • Bolus injection of 0.75 mg/kg over 2 minutes IV or via arterial sheath just prior to the PCI procedure, followed by IV infusion of 1.75 mg/kg/hour (or per local label depending on renal function) until completion of the procedure • Infusion discontinued upon completion of the PCI procedure Bivalirudin Arm • Aspirin + P2Y12-Inhibitor for all patients prior to PCI. • GPI could be used only provisionally for procedural or angiographic complications ENDPOINTS (Assessed at 3 and 30 Days) Primary Efficacy Endpoint Primary Safety Endpoint Secondary Endpoints • Composite of death, non-fatal MI, non-fatal stroke and urgent TLR through Day 3. • Incidence of bleeding (BARC 3 or 5; not related to CABG) through Day 3; • Components of the primary endpoint through day 3 • Composite of death, non-fatal MI, non-fatal stroke and urgent TLR through day 30 • Bleeding endpoints through day 30 • Incidence and severity of allergic adverse events. STATISTICAL ANALYSIS • Efficacy analyses were based upon the intention-to-treat population, with the test of the null hypothesis based on the odds ratio and two-sided 95% CI from the CochranMantel-Haenszel test with risk subgroup (Subgroup A, B, or C) as the stratification factor. • Superiority Trial Design with an expected risk reduction of 20% for the primary efficacy endpoint. • • Anticipated 830 adjudicated events, providing an 90% power for a two-sided alpha less than or equal to 0.049 with one planned interim efficacy review at 50% enrollment. Endpoint Estimations: Primary endpoint event rate of 7.0% in the Bivalirudin arm (8% in Subgroup A, 6% in Subgroups B and C) Primary endpoint event rate of 5.6% in the REG1 arm. Estimated sample size of 13,200 patients, of whom at least 6600 were to be enrolled from Subgroup A. Secondary endpoints were to be evaluated using a hierarchical closed testing procedure to preserve overall Type I error. REGULATE PCI Enrollment September 13, 2013 • Initial recruitment in the trial April 2, 2014 • Enrollment expanded to include patients in Subgroup A after review of safety among the first approximately 1000 patients. June 29, 2014 • Ongoing evaluation of reports of severe allergic reactions • Sponsor and executive committee suspended enrollment • A total of 3232 of the planned 13,200 patients had been enrolled at 225 hospitals in North America and Europe. August 21, 2014 • DSMB recommended permanent termination of the trial based on findings of excess rates of allergic reactions with REG1 without evidence of offsetting benefit. Top 5 Enroller Countries Country 1 2 3 4 5 United States Canada Estonia Italy Slovakia 17 Participating Countries N. Of Patients 1965 288 174 131 124 Participating Countries Top 5 Enroller Centers Country Investigator 1 United States J .Tauth 2 United States G. Soliman 3 Estonia T. Marandi 4 Canada W. Cantor Center HS Cardiology Associate (Hot Springs National Park, AR) Heart Center, Inc. (Huntsville, AL) University of Tartu (Tartumaa, Eesti) Southlake Regional Health Centre, N. Of Patients 304 148 134 123 (Newmarket, ON) 5 Slovakia M. Hranai Národný, Oddelenie Intervenčnej Kardiológie 123 STUDY CONSORT DIAGRAM BASELINE CHARACTERISTICS REG1 (N = 1616) Bivalirudin (N = 1616) Age - mean, years 65 +/- 11 65 +/- 11 Male sex – no. (%) 1215 (75) 1184 (73) Diabetes mellitus – no. (%) 571 (35) 553 (34) Body mass index – mean, kg/m2 30 +/- 6 30 +/- 6 Prior myocardial infarction – no. (%) 576 (36) 582 (36) Prior PCI – no. (%) 818 (51) 850 (53) Prior coronary bypass surgery – no. (%) 278 (17) 265 (16) 67 (4) 68 (4) Left ventricular dysfunction (EF <55%) – no. (%) 553 (38) 594 (41) Current tobacco use – no. (%) 348 (22) 322 (20) History of any allergies – no. (%) 520 (32) 538 (33) Subgroup A 246 (15) 247 (15) Subgroup B 1101 (68) 1100 (68) Subgroup C 269 (17) 269 (17) Characteristic Prior stroke – no. (%) Randomization stratification subgroup PROCEDURAL CHARACTERISTICS REG1 Bivalirudin (N = 1616) (N = 1616) 1186 (73) 1223 (76) Clopidogrel – no. (%) 891 (55) 952 (59) Ticagrelor – no. (%) 178 (11) 173 (11) Prasugrel – no. (%) 126 (8) 110 (7) Taken after PCI – no. (%) 1595 (99) 1584 (98) Clopidogrel – no. (%) 1105 (68) 1134 (71) Ticagrelor – no. (%) 285 (18) 268 (17) Prasugrel – no. (%) 231 (14) 207 (13) Taken within 48 hours prior to randomization – no. (%) 1553 (96) 1544 (96) Taken after PCI – no. (%) 1599 (99) 1589 (99) Bailout GP IIb/IIIa inhibitor – no. (%) 20 (1) 19 (1) Bailout heparin – no. (%) 5 (0) 2 (0) Drug eluting stent used only – no. (%) 1224 (81) 1217 (81) Vascular closure device used – no. (%) 512 (32) 493 (31) Characteristic Platelet P2Y12 antagonist Taken within 48 hours prior to randomization – no. (%) Aspirin Procedural anticoagulants PCI ACCESS SITE Stent Used During PCI Platelet P2Y12 Antagonist Therapy After PCI • 99% treated with Aspirin in both randomization arms EFFICACY ENDPOINTS (Day 3) *Composite of death, myocardial infarction, stroke, or urgent target lesion revascularization EFFICACY ENDPOINTS Day 30 P = 1.00 P = 0.69 P = 0.06 P = 0.36 P = 0.71 P < 0.01 BLEEDING SAFETY ENDPOINTS Bleeding Rates by Day 3 *Major Non-CABG Bleeding (BARC Types 3 or 5) Bleeding Rates by Day 30 ALLERGIC EVENTS REG1 (N = 1605) Bivalirudin (N = 1601) 10 (0.6) 1 (< 0.1) Fatal Event 1 0 Severe Event (Anaphylactic Reaction) 9 1 Mucocutaneous 9 1 Respiratory 8 1 Circulatory 6 1 GI or GU 4 0 14 (0.9) 9 (0.5) Severe Event (Anaphylactic Reaction) 8 3 Non-Severe Event 6 6 End Point by Day 3 Serious Allergic Events Organ System Involvement Non-Serious Allergic Events Subgroup Analysis Primary Efficacy Endpoint and Major Bleeding No significant interactions in the primary efficacy and safety endpoint LIMITATIONS • Given the early termination of the trial with only 211 of the planned 830 primary endpoint events accrued, any conclusion regarding the safety in bleeding and efficacy in ischemic events of REG1 compared with Bivalirudin has to be considered exploratory. • Open label design- Independent CEC blinded to treatment allocation was put forth to minimize bias in endpoint adjudication. CONCLUSIONS • The reversible factor IXa inhibitor REG1, as currently formulated, is associated with an infrequent but unacceptably high rate (0.6%) of severe allergic reactions. • In patients undergoing PCI, the REG1 Anticoagulation System was associated with a similar incidence of ischemic events, but a higher rate of moderate/severe bleeding compared to Bivalirudin monotherapy. • The concept of high-level anticoagulation with active reversal is promising, however its clinical role has yet to be defined and further improvements are needed in its safety profile. • Future investigations are planned to identify the mechanism of allergic reactions associated with REG1 Anticoagulation System. Thank you for your attention 0 Sheath Removal REG1 Anticoagulation System • Sheath was removed approximately 2-10 minutes after anivamersen reversal Bivalirudin Sheath removal per: • Femoral access without VCD: approximately 2-4 hours from the completion of PCI procedure • Femoral access with VCD: at the completion of the PCI procedure • Radial access: at the completion of the PCI procedure Select Exclusion Criteria • Acute ST-segment elevation myocardial infarction within 48 hours • Use of Fibrinolytic agents within 48 hours, GP IIb/IIIa inhibitors within 24 hours or planned use during PCI, Bivalirudin within 24 hours • Planned staged PCI, CABG or valve surgery within 30 days after randomization • Known allergy to study medication