* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Time of origin and distribution of a new cell type in the rat cerebellar
Survey
Document related concepts
Transcript
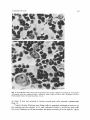
Exp. Brain Res. 29, 265-274 (1977) Experimental Brain Research 9 Springer-Verlag1977 Time of Origin and Distribution of a New Cell Type in the Rat Cerebellar Cortex J. Altman and S.A. Bayer Laboratory of Developmental Neurobiology, Department of Biological Sciences, Purdue University, West Lafayette, Indiana 47907, USA Summary. A new cell type was identified in the granular layer of the rat cerebellum. It has a lightly staining nucleus with a nucleolus, it is spherical in shape and is larger than granule cells and smaller than Golgi cells. Thesepale cells are preferentially concentrated in the nodulus, the ventral uvula, the lingula, the flocculus, and parts of the paraflocculus. According to autoradiographic (3H-thymidine) evidence, over 60 % of these pale cells are formed on embryonic days 19 and 20, and their production comes to an end soon after birth, prior to the differentiation of granule cells. The possible relation of pale cells to vestibular afferents of the cerebellar cortex is discussed. Key words: Cerebellum - Pale cells - Vestibular afferents Introduction When pregnant rats are injected with 3H-thymidine between gestation days 17 and 20 (E17-20) to tag multiplying cells in the cerebellum, neither the Purkinje cells nor the small interneurons of the granular and molecular layers are labelled in histological autoradiograms of the mature offspring. The Purkinje cells are not labelled because their formation ends by day E l 6 (Das and Nornes, 1972; Schultze et al., 1974; our observations) whereas, the basket, stellate and granule cells are either unlabelled or have few grains over their nuclei because there is considerable label dilution due to repeated multiplication of their precursor cells until after birth (Altman, 1966, 1969; Altman and Das, 1966). However, in rats injected between days E17 and birth we noticed many intensely labelled cells in the granular layer of the nodulus, and some in other lobules. These labelled cells were larger than granule cells but much smaller than Golgi cells, and typically had pale nuclei. In adjacent, nonautoradiographic sections similar pate cells with visible nucleoli were present in considerable number in the nodulus. The purpose of this study was to determine the identity and distribution of these pale cells with light microscopy and autoradiography. Counts were made 266 J. Altman and S.A. Bayer o f t h e s e cells in h i s t o l o g i c a l s e c t i o n s i n t h e n o d u l u s a n d c o n t r a s t e d w i t h c o u n t s o b t a i n e d i n t h e declive. T h e n u m b e r of p a l e cells s e e n i n h i s t o l o g i c a l s e c t i o n s was c o r r e l a t e d w i t h t h e n u m b e r o f i n t e n s e l y l a b e l l e d cells in a d j a c e n t a u t o r a d i o g r a m s . I n t h e s a m e l o b u l e s P u r k i n j e cells a n d G o l g i cells w e r e also c o u n t e d to allow us to e x p r e s s t h e r e s u l t s as ratios. F i n a l l y , i n a g r o u p of a n i m a l s all i n t e n s e l y l a b e l l e d cells w e r e c o u n t e d i n t h e 10 v e r m a l l o b u l e s o f L a r s e l l ( 1 9 5 2 ) a n d t h e n u m b e r of p a l e cells w e r e e x p r e s s e d p e r u n i t a r e a o f t h e g r a n u l a r layer. Material and Methods We used our collection of 3H-thymidine autoradiograms of the brains of adult rats. In the embryonic series (E) of this collection sperm-positive (gestational or embryonic day 1; El) Pnrdue-Wistar females were injected subcutaneously with two successive daily doses of 3H-thymidine (specific activity 6.0 C/mM; dose, 5 ~c/g body weight) at the following overlapping gestational ages: E l 3 + 14, E14+ 15 . . . . E21+22. In the postnatal (P) series, infants were injected with the same dose beginning at birth with two successive doses on overlapping days; use was made here of rats injected on days P0+ 1 and P 1+ 2. All animals were killed on day P60 by cardiac perfusion with 10 % neutral formalin. The brains were embedded in paraffin, sections were cut at 6 p~mserially in the three planes and every 15th section was saved. Successive sections were stained with cresyl violet and hematoxylin-eosin for light microscopy or prepared for autoradiography. The latter procedure was described elsewhere (Altman, 1964). Briefly, deparaffinized sections were coated with Kodak NTB-3 emulsion in the dark, exposed for 90 days with a dessicant, developed with D19, and stained with hematoxylin-eosin. In this investigation we used only sagittally cut, matched sections of the cerebella of male rats (6 cerebella per injection group). Cell counting was done microscopically at 625 x magnification with the aid of an ocular grid. Lobular areas were determined with a high-resolution projection system integrated with a Summagraph X-Y digitizer and a Wang 2200 computer. The estimation of the proportion of cells differentiating (ceasing to divide) on a particular day utilized the progressively delayed cumulative labelling procedure (Bayer and Altman, 1974). The rationale of the method is that as long as practically all the cells of a selected brain region can be labelled (in the population studied here this can be accomplished with two successive daily injections) all the labelled cells are considered to have their precursors multiplying, that is, they have not started to differentiate. When as a result of a delay in the onset of injections some cells can no longer be labelled, the proportion of cells that cannot be labelled as a result of a single day delay is considered the comp!ement that differentiated on the previous day. For instance, the cells differentiating on day El7 are determined as follows: El7 = ( e l 7 + 18) - (El8+ 19). Results Regional Distribution of Pale Cells F i g u r e 1 i l l u s t r a t e s this h i t h e r t o n e g l e c t e d cell t y p e i n t h e g r a n u l a r l a y e r . It t e n d s to h a v e a spherical, l i g h t l y s t a i n i n g n u c l e u s w i t h a n i d e n t i f i a b l e n u c l e o l u s . I n a d e q u a t e l y p r e p a r e d m a t e r i a l p a l e cells ( p r e d o m i n a n t l y 7 - 8 ~ m ) a r e easy to d i s t i n g u i s h f r o m t h e s m a l l e r ( p r e d o m i n a n t l y 4 - 5 ~tm) a n d d a r k l y s t a i n i n g g r a n u l e cells o r t h e larger, p o l y m o r p h o u s a n d b e t t e r s t a i n i n g G o l g i cells. T e n t a t i v e l y we shall call t h e m p a l e cells. I n t h e n o d u l u s p a l e cells w e r e s e e n s i n g l y b u t m o r e o f t e n t h e y t e n d e d to c l u s t e r , r e g i o n s c o n t a i n i n g m a n y p a l e cells a l t e r n a t i n g w i t h r e g i o n s t h a t h a d few Cerebellar Pale Cells 267 Fig. 1. A and B Pale cells (arrows) in the granular layer of the nodulus of an adult rat. Also shown are granule cells (gr), glomeruli (gl), a relatively small Golgi cell (Go), and a Purkinje cell (Pu). Hematoxylin-eosin, oil immersion. Bar, 10 ~tm o r n o n e . It was n o t u n u s u a l to l o c a t e s e v e r a l p a l e cells a r o u n d a g l o m e r u l u s (Fig. 1). C o u n t s of p a l e , P u r k i n j e a n d G o l g i cells in m a t c h e d , m i d s a g i t t a l sections in t h e n o d u l u s a n d t h e d e c l i v e in 12 r a t s i n d i c a t e d ( T a b l e 1) a b o u t two p a l e ceils for e v e r y P u r k i n j e cell in t h e n o d u l u s , t e n t i m e s as m a n y as in t h e declive. T h e r e 268 J. Altman and S.A. Bayer Table 1. Comparison of the Distribution of Pale Cells with Respect to Purkinje Cells and Golgi Cells in the Nodulus (Lobule X) and Part of the Declive (Lobule VIb) a) Cell Counts Pale Cells (with nucleoli) Purkinje Cells (with nucleoli) Golgi Cells (with nucleoli) b) Ratios Pale Cells/Purkinje Cells Golgi Cells/Purkinje Cells Pale Cells/Golgi Cells Nodulus (X) Declive (VIb) 128.2 + 36.45 61.9 + 13.84 17.6 + 5.93 7.1 + 5.13 28.9 _+7.19 5.8 + 2.4 2.0 0.3 7.3 0.2 0.2 1.2 Fig. 2. A Intensely labelled cells (arrows) in the granular layer of the nodulus of an adult rat injected with 3H-thymidine on days E19+20. Purkinje cells (bottom) are unlabelled because they differentiated before the injection; granule cells that differentiate post-natally are lightly labelled. B A comparable nonautoradiographic section with several pale cells (arrows). Bar, 20 ~xm Cerebellar Pale Cells 269 Fig. 3. A Low power photomicrograph of the nodulus in a rat injected on days E19+20. Note the high concentration of labelled cells. Bar, 200 ~tm.B Higher magnification of the area outlined in A. Bar, 100 ~xm.C Intensely labelled cells are scarce in the declive (from the same autoradiographic section as A). Bar, 100 ~tm were only small differences in the relative frequency of Golgi cells in the same regions. Distribution of Labelled Cells as a Function of Age at Injection Intensely-labelled cells (Fig. 2A) were counted in the nodulus and the declive in rats that were injected on the following days: E 1 7 + 1 8 ; E 1 8 + 1 9 ; E 1 9 + 2 0 ; E 2 0 + 21; E21 + 22; P 0 + 1 and P1 + 2. Six cerebella were available per injection group (a group with E 2 2 - P 0 injection was not available). In the nodulus m a n y intensely labelled cells were present in the E 1 7 + 1 8 group. Maximal and comparable numbers of labelled cells were obtained in the E 1 8 + 1 9 and E 1 9 + 2 0 groups (Fig. 3A, B) with m e a n counts of 126 and 132 cells, respectively (Fig. 4). The latter values closely matched the n u m b e r of pale cells counted in adjacent, non-autoradiographic sections in the same 12 animals (128 cells; Table 1). This supports the identity of pale cells as the intensely 270 J. Altman and S.A. Bayer # 130120I10I00 90 80Fig. 4. Empty bars show the mean number of intensely labelled cells counted in the nodulus in the injection groups indicated above the bars. Solid bars show the calculated percentage of cells formed on the days indicated along the abscissa. Because an E22+P0 group was not available, the solid bar (with arrow) for E21 indicates all the cells formed on days E21 and thereafter 7060 5040 30 20I0El7 El8 El9 E20 E21 labelled cells obtained during this period. The n u m b e r of well-labelled cells progressively declined in the subsequent injection groups (Fig. 4) and only an occasional pale cell was labelled in the P0-1 group. Calculations with the previously described method of progressively delayed cumulative labelling indicated that the precursors of pale cells did not start to differentiate (cease to divide) until day E l 9 , since the entire population could be labelled in the E 1 9 + 2 0 group. It was estimated (Fig. 4) that 4 0 % of the cells differentiated on day E l 9 and 2 2 % on day E20. The remaining 3 8 % of pale cells (the proportion that could be labelled in the E21 + 22 group) differentiated between day E21 and the first 2 days after birth. (Daily estimates could not be m a d e because an E 2 2 - P 0 group was not available.) In contrast to the nodulus, there were relatively few labelled pale cells at any injection ages in the declive. In the E l 8 + 19 group the m e a n cell count was 9.2, and in the E 1 9 + 2 0 group it was 8.6. These low values were comparable to the m e a n n u m b e r of pale cells counted in adjacent non-autoradiographic sections (7.1 cells; Table 1). Regional Distribution of Labelled Pale Cells The midsagittal vermis was subdivided (Fig. 5) according to Larsell (1952). Intensely labelled cells were counted separately in the ten lobules in the E 1 8 + 1 9 and E 1 9 + 2 0 injection groups, the groups that provided maximal Cerebellar Pale Cells 271 VIa Vl b .v Fig. 5. Tracing of midsaginal vermis with lobular subdivisions (I-X) according to Larsell (1952). Dots delineate the boundaries of the lobules for the areal determinations; the numbers inside the lobules are the mean counts of labelled pale cells in two injection groups (E18+ 19 and E19+20) I • Table 2. Mean Number of Labelled Pale Cells in Matched Parasagittal Sections in the 10 Vermal Lobules of Larsell (I-X) in the E18+19 and E19+20 Injection Groups (11 animals), with an Estimate of Cell Number/mm 2 of Granular Layer I II III IV V VI VII VIII IX X Total a) Mean Cells/Lobule 16 16 17 12 9 7 10 9 70 122 288 b) Granular Layer Area in mm2 0.358 0.558 0.734 0.650 0.869 1.136 0.484 0.669 1.318 0.649 7.425 c) Estimated Cell/mm2 of Granular Layer 45 29 23 18 10 6 21 13 53 188 406 (Fig. 4) a n d a p p a r e n t l y c o m p r e h e n s i v e l a b e l l i n g of p a l e cells ( T a b l e 1). A s a c o r r e c t i o n for t h e d i f f e r e n t d i m e n s i o n o f t h e lobules, t h e m e a n a r e a o f t h e g r a n u l a r l a y e r o f e a c h l o b u l e was also d e t e r m i n e d in t h e s a m e sections w i t h c o m p u t e r aid. T h e results ( T a b l e 2) i n d i c a t e t h a t in t h e p a r a s a g i t t a l s e c t i o n s s a m p l e d o v e r 4 0 % o f t h e p a l e cells a r e c o n c e n t r a t e d in t h e n o d u l u s . T h e r e is also a m o d e r a t e l y high c o n c e n t r a t i o n in t h e u v u l a ( l o b u l e I X , p r e d o m i n a n t l y in t h e v e n t r a l uvula) a n d in t h e l i n g u l a ( l o b u l e 1). O t h e r r e g i o n s o f t h e c e r e b e l l u m w e r e n o t e v a l u a t e d q u a n t i t a t i v e l y , b u t a r e l a t i v e l y high c o n c e n t r a t i o n o f i n t e n s e l y l a b e l l e d cells w e r e n o t e d in t h e flocculus a n d an a b o v e a v e r a g e c o n c e n t r a t i o n in p a r t s o f t h e p a r a f l o c c u l u s . Discussion T h e i d e n t i f i c a t i o n o f p a l e cells was a i d e d b y 3 H - t h y m i d i n e a u t o r a d i o g r a p h y . B e c a u s e t h e s e cells a r e f o r m e d d u r i n g a p e r i o d w h e n t h e r e is a lull in c e r e b e l l a r n e u r o g e n e s i s , t h e y t e n d to " l i g h t u p " in a u t o r a d i o g r a m s for e a s y i d e n t i f i c a t i o n 272 J. Altmanand S.A. Bayer as an, at least developmentally, unique class of cells. The quiescence in cerebellar neurogenesis between day E l 7 and birth is probably due to the fact that the external germinal layer (which is the source of the postnatally-forming basket, stellate and granule cells) begins to spread from the posteroventra! aspect of the cerebellum over its surface on day E17 and does not reach its anteroventral aspect until day E21 (Altman and Bayer, in preparation). Since the external germinal layer is only in the process of formation when most of the pale cells are differentiating, it is likely that pale cells (like Purkinje cells and probably Golgi cells) arise in the germinal neuroepithelium of the cerebellar recess of the fourth ventricle. In autoradiograms the opaque grains often obscure the cell nucleus. Therefore, the identification of all intensely labelled cells, in the injection groups referred to, as pale cells was not possible. But the hypothesis of their identity was reinforced by the comparable absolute counts obtained, and by their similar differential Concentration in the nodulus and the declive. Since pale cells have not been recognized with the Golgi technique and electron microscopy, we cannot make definitive statements whether they are neurons or glia. Our belief that they are neurons is based on the fact that they have a prominent nucleolus and, more importantly, that they have a restricted distribution in the cerebellar cortex. The regions where pale cells were encountered in high or moderate concentration (nodulus, ventral uvula, lingula and flocculus) are the lobules that receive afferents from the labyrinth and the vestibular nuclei (Dow, 1939; Brodal and Hoivik, 1964; Precht and Llinfis, 1969; Carpenter et al., 1972; Wilson et al., 1974; Shinoda and Yoshida, 1975). This suggests some relationship between pale cells and the vestibulocerebellum. Vestibular afferents terminate in the cerebellum as mossy fibers (Llinfis et al., 1971; Carpenter et al., 1972; Wilson et al., 1974; Shinoda and Yoshida, 1975). Brodal (Brodal and Drablos, 1963; Brodal, 1972) recognized a special type of mossy fiber ending, referred to as the "nodular" type, in the nodulus, flocculus, the ventral paraflocculus, and the lingula, with occasional ones elsewhere. Brodal suggested that they are endings of vestibular afferents. The possibility that these "nodular" mossy fibers end in relation to pale cells (in addition to or in place of granule cells) is intriguing. Wilson et al. (1972, 1974) made recordings in the vestibulocerebellum of pigeons in response to stimulation of the labyrinth. They identified in the granular layer a class of short-latency units, called G units, that were in some instances fired from a single ampulla. Although Wilson and his collaborators suggested that their sample of G units represented mossy fiber endings, and included granule cells and Golgi cells, it is again tempting to speculate that these units are related to pale cells. Most of the cells of the cerebellar cortex have been known for over a century (Palay and Chan-Palay, 1974) and it is surprising to discover a new cell type after so many years. Perhaps the poor stainability of these cells, partial resemblance to astrocytes, and restricted distribution may be some of the reasons why they were neglected. More likely, they may have been seen but classified as Golgi cells. Dogiel in 1896 (quoted from Larsell, 1967) referred to small Golgi cells in birds that have both dendrites and axons confined to the granular layer. Cerebellar Pale Cells 273 Ram6n y Cajal (1911) described several types of "stellate" cells in the granular layer, including small ones. Indeed, pale cells have many features in common with "small Golgi cells" recently described by Palay and Chan-Palay (1974) without reference to their preferential vestibulocerebellar distribution. Brodal and Dabl~s (1963) and Llinfis and Hillman (1969) remarked on an unduly high number of Golgi cells in the vestibulocerebellum; but this was not borne out by our counts. Since pale cells (which may be Golgi type II cells) bear little resemblance to and have a different distribution than the specific Golgi cells, it may be heuristic to classify them as a discrete cell type. Their physiological characterization, and morphological description with the Golgi technique and electron microscopy could be worthwhile undertakings. Acknowledgements. This research was supported by the National Institute of Mental Health and the Energy Research and Development Administration. We thank Sharon Evander, Paul Lyons, Patricia McNerney, Harriet Sheets and Larissa Gilham for their technical assistance. References Altman, J.: The use of fine-resolution autoradiography in neurological and psychobiological research. In: Response of the Nervous System to Ionizing Radiation (eds. T.J. Haley and R.S. Snider), pp. 336-359. Boston: Little, Brown 1964 Altman, J.: Autoradiographic and histological studies of postnatal neurogenesis, lI. J. comp. Neurol. 128, 431-474 (1966) Altman, J.: Autoradiographic and histological studies of postnatal neurogenesis. III. J. comp. Neurol. 136, 269-294 (1969) Altman, J., Das, G.D.: Autoradiographic and histological studies of postnatal neurogenesis. I. J. comp. Neurol. 126, 337-390 (1966) Bayer, S.A., Altman, J.: Hippocampal development in the rat: Cytogenesis and morpbogenesis examined with autoradiography and low-level X-irradiation. J. comp. Neurol. 158, 55-80 (1974) Brodal, A.: Vestibulocerebellar input in the cat: anatomy. Progr. Brain Res. 37, 315-327 (1972) Brodal, A., Drabl~s, P.A.: Two types of mossy fiber terminals in the cerebellum and their regional distribution. J. comp. Neurol. 121, 173-187 (1963) Brodal, A., Hoivik, B.: Site and mode of termination of primary vestibulo-cerebellar fibers in the cat. An experimental study with silver impregnation methods. Arch. ital. Biol. 102, 1-21 (1964) Carpenter, M.B., Stein, B.M., Peter, P.: Primary vestibulocerebellar fibers in the monkey: Distribution of fibers arising from distinctive cell groups of the vestibular ganglia. Amer. J. Anat. 135, 221-250 (1972) Das, G.D., Nornes, H.O.: Neurogenesis in the cerebellum of the rat: an autoradiographic study. Z. Anat. Entwickl.-Gesch. 138, 155-165 (1972) Dow, R. S.: Cerebellar action potentials in response to stimulation of various afferent connections. J. Neurophysiol. 2, 543-555 (1939) Larsell, O.: The morphogenesis and adult pattern of the lobules and fissures of the cerebellum of the white rat. J. comp. Neurol. 97, 281-356 (1952) Larsell, O.: The Comparative Anatomy and Histology of the Cerebellum from Mysinoids through Birds. pp. 270-273. Minneapolis: Univ. Minnesota Press 1967 Llinfis, R., Hillman, D.E.: Physiological and morphological organization of the cerebellar circuits in various vertebrates. In: Neurobiology of Cerebellar Evolution and Development (ed. R. Llinfis), pp. 43-73. Chicago: Amer. Med. Assoc. 1969 Llinfis, R., Precht, W., Clarke, M.: Cerebellar Purkinje cell responses to physiological stimulation of the vestibular system in the frog. Exp. Brain Res. 13, 408-431 (1971) Palay, S.L., Chan-Palay, V.: Cerebellar Cortex: Cytology and Organization. pp. 121-123. Berlin: Springer 1974 274 J. Altman and S.A. Bayer Precht, W., Llingts, R.: Functional organization of the vestibular afferents to the cerebellar cortex of frog and cat. Exp.. Brain Res. 9, 30-52 (1969) Ram6n y Cajal, S.: Histologie du Syst~me Nerveux de l'Homme des Vert6br6s. Vol. 2, pp. 1-54. Paris: Maloine 1911 Schultze, B., Nowak, B., Maurer, W.: Cycle times of the neural epithelial cells of various types of neuron in the rat. An autoradiographic study. J. comp. Neurol. 158, 207-218 (1974) Shinoda, Y., Yoshida, K.: Neural pathways from the vestibular labyrinths to the flocculus in the cat. Exp. Brain Res. 22, 97-111 (1975) Wilson, V.J., Anderson, J.A., Felix, D.: Semicircular canal input to pigeon vestibulocerebellum. Brain Res. 45, 230-235 (1972) Wilson, V.J., Anderson, J.A., Felix, D.: Unit and field potential activity evoked in the pigeon vestibulocerebellum by stimulation of individual semicircular canals. Exp. Brain Res. 19, 142-157 (1974) Received February 1, 1977