* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download calculations of mechanical work in 1, 2 and 3 dimensions
Atomic theory wikipedia , lookup
Vapor-compression refrigeration wikipedia , lookup
Water pollution wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Thermal spraying wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Thermodynamics wikipedia , lookup
Transition state theory wikipedia , lookup
History of electrochemistry wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Double layer forces wikipedia , lookup
Surface tension wikipedia , lookup
Molecular dynamics wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Vibrational analysis with scanning probe microscopy wikipedia , lookup
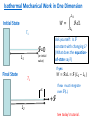
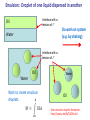
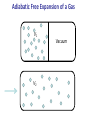
PHY1039 Properties of Matter Thermodynamic Mechanical Work: 1, 2 and 3 Dimensions (See Finn’s Thermal Physics, Ch. 2) 27 February and March 1 Lectures 7 and 8 Types of Work Mechanical: related to force acting through a distance: our main focus Electrical: electrical current flowing through a resistor under an applied potential Capacitive: storing of electric charge under an applied electric field Magnetic: increasing the magnetic moment of a material with an applied magnetic field SI Units of work: Nm (also called a Joule, J) http://www.nearfield.com/~dan/sports/bike/river/coyote/index.htm Examples of Mechanical Work Actuator driven by thermal expansion of water and air Force acting through a distance to do work on surroundings Change in length, L, under a constant (?) force Source: www.ritsumei.ac.jp Front cover of Scientific American, Oct. 2003 Work can be done on the system by the surroundings or done by the system on the surroundings. Example of negative linear expansivity being exploited to convert chemical energy into work A chemical reaction with methanol creates heat in the NiTi alloy wire. At a higher temperature, the wire’s length shrinks - thus lifting a weight and doing work on the surroundings. V.H. Ebron et al., Science (2006) 311, 1580 Mechanical Work, W, in One Dimension 𝑑𝑊 = F𝑑𝐿 Initial State 𝐿2 𝑊= T1 F 𝑑𝐿 𝐿1 +F L1 Ask yourself: Is F constant with changing L? If yes: 𝑊 = F𝐿 = F (𝐿2 − 𝐿1 ) Final State T2 > T1 IMPORTANT Sign Convention: dL +F L2 Usually dL is +ve when T increases! Positive W: work done on system by surroundings Negative W: work done on surroundings by the system Isothermal Mechanical Work in One Dimension 𝐿2 𝑊= Initial State 𝐿1 T1 F=0 L1 F 𝑑𝐿 (or initial value) Ask yourself: Is F constant with changing L? (What does the equationof-state say?) If yes: Final State 𝑊 = F𝐿 = F (𝐿2 − 𝐿1 ) T1 dL +F If no: must integrate over F(L). L2 See today’s tutorial. Weak Nano-scale Forces Can be Measured Atomic force microscope (AFM) The AFM probe is exceedingly sharp so that only a few atoms are at its tip! Sensitive to forces on the order of nano-Newtons. Tip for Atomic Force Microscopy F The tip is on a cantilever, which typically has a spring constant on the order of k = 10 N/m. Radius of curvature ~ 10 nm Ideally, one of the atoms at the tip is slightly above the others. Modelled as a simple spring: F = kz where z is the deflection in the vertical direction. AFM tips from NT-MDT. See www.ntmdt.ru Doing Work on the Nano-Scale Force /nN F 200 Pushing on AFM probe tip 100 trace retrace 0 -100 -200 Pulling on the AFM probe tip -300 -400 0 2 4 Distance/ m 6 8 L Emulsion: Droplet of one liquid dispersed in another Interface with a tension of G Oil Do work on system (e.g. by shaking) Water Interface with a tension of G Oil Water Water Work to create emulsion droplets: 𝐴 𝑊= Γ𝑑𝐴 𝐴𝑜 Oil See emulsion droplet formation: http://youtu.be/9y7J3CBe1eI Physics of a “Bubble Jet” Printer Question for Tutorial: How much work is required to create a droplet of ink? Isothermal Mechanical Work in Two Dimensions Initial State ℓ For each surface: T1 F Γ= ℓ A1 𝑑𝑊 = F𝑑𝐿 = Γℓ𝑑𝐿 𝑑𝑊 = Γ𝑑𝐴 𝐴2 𝑊= 𝐴1 L Final State ℓ A2 G𝑑𝐴 Membrane: two sides Surface: one side only T1 dA dL F Ask yourself: Is G constant with changing A? If yes: 𝑊 = G𝐴 = G(𝐴2 − 𝐴1 ) Surface Tension of Molecular Layers Langmuir trough water G G is a function of A! http://www.engineerdir.com/product/catalog/ 16626/ Area adjusted with barriers barrier A http://www.augsburg.edu/ppages/~stottrup/ http://www.reflec.ameslab.gov/aboutus/monolayers.html Exptech.html Isobaric Mechanical Work in Three Dimensions Initial State F Isobaric = constant P A A V1 F 𝐴𝑝𝑝𝑙𝑖𝑒𝑑 𝑓𝑜𝑟𝑐𝑒 P= = 𝐴 T1 Final State F A A V2 +dL 𝑑𝑊 = −𝑃𝑑𝑉 𝐴𝑟𝑒𝑎 Positive P is pressing inward. But F pressing inwards is negative! Only applies to reversible processes! T2 > T1 𝑑𝑊 = F𝑑𝐿 = −𝑃𝐴𝑑𝐿 𝑉2 𝑊=− 𝑃𝑑𝑉 𝑉1 Ask yourself: Is P constant with changing V? If yes: 𝑊 = −𝑃𝑉 = −𝑃(𝑉2 − 𝑉1 ) Question: Is this result consistent with our sign convention? Isobaric Conditions Earth’s Atmospheric Pressure Height (km) Crab Nebula Pressure is low but roughly constant! 0 1 2 3 4 5 6 7 8 9 10 Pressure (kPa) 101.3 89.9 79.5 70.1 61.6 54.0 47.2 41.1 35.6 30.7 26.4 http://en.wikipedia.org/wiki/File:Crab_Nebula.jpg Isochoric = constant volume. How much work is done in an isochoric process? Isothermal Mechanical Work in Three Dimensions Initial State Isothermal = constant T A A 𝑉2 T1 𝑊=− 𝑉1 V1 Apply a pressure to the system and compress it to a smaller V. Final State FA A V2 𝑃𝑑𝑉 -dL T1 Ask yourself: Is P constant with changing V? (What does the equation-ofstate say?) Work can be done on solid, liquid and gas systems by compressing them. Work is done on the surroundings by a system when it expands. CO Map of the Universe This is the first-ever all-sky map of carbon monoxide in the cosmos. The Planck space telescope was designed to look at the background glow in the cosmos in an effort to understand how it formed. Coincidentally, scientists have found that it can help to spot star-forming regions where carbon monoxide glows brightly despite its low abundance http://www.bbc.co.uk/news/science-environment-17027949 Adiabatic Free Expansion of a Gas V1 V2 Vacuum Compressive (Tensile) Force from Thermal Expansion of Beams Applying the “Thermodynamic Method” to Problem Solving Wire pulled in tension between two walls: T1 F1 F1 L1 Question: What is the increase on the tension of the wire when it is cooled from a temperature of T1 to T2? (F1, L1, T1) (F2, L1, T2)