* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Help With Coming Off CPB - Society of Cardiovascular
Survey
Document related concepts
History of invasive and interventional cardiology wikipedia , lookup
Electrocardiography wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Jatene procedure wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Coronary artery disease wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Ventricular fibrillation wikipedia , lookup
Transcript
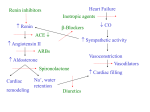
Help With Coming Off CPB: Deciding what inotropes to use Alina Nicoara, MD Department of Anesthesiology Yale University School of Medicine The profile of the patients undergoing cardiac surgery has changed over the past decade as well as the complexity of the cardiac surgery procedures. Cardiac surgery is performed in an increasingly aging population with complex coexisting pathology, reoperations and emergency surgery are more frequent and the incidence of compromised ventricular function has increased. These patients are at risk for tenuous separation from CPB and subsequent deterioration into a low cardiac out syndrome (LCOS) associated with higher mortality rates [1]. Myocardial dysfunction resulting in LCOS can occur secondary to events related to cardiac surgery and cardiopulmonary bypass both in patients with abnormal and normal preoperative ventricular function [2, 3]. Despite a wide range of available inotropic agents, there is no consensus regarding the treatment of LCOS post-CPB and the anesthesiologist can be faced with difficult decisions at the time of separation from CPB. Physiologic changes related to CPB Acute myocardial dysfunction can occur postCPB, even in conditions of adequate surgical repair and revascularization. This myocardial stunning may cause transient systolic and diastolic dysfunction despite normal or near-normal oxygen supply and is associated with increased morbidity and mortality [4]. It can be due to ischemia during aortic crossclamping, inadequate myocardial protection with cardioplegic solutions, hypothermia with cardioplegic and topical solutions and incomplete rewarming, coronary microemboli of air or particulate matter, myocardial edema from surgical manipulation, and continuous reperfusion injury [3]. This contractile dysfunction may occur in patients with normal preoperative systolic function, typically peaks between 4 and 6 hours after surgery and usually resolves around 24 hours postoperatively [3]. The return to baseline function may be delayed in patients with preoperative ventricular dysfunction [3]. The underlying pathophysiology in myocardial stunning may be related to impaired calcium handling by the sarcoplasmic reticulum resulting in decreased efficiency of energy utilization and increased myocardial oxygen consumption, a decrease in the myofilament sensitivity to calcium and less efficient oxygen use by the contractile apparatus with an increased myocardial oxygen consumption for excitation-contraction coupling [5-7]. Other proposed mechanisms responsible for reperfusion injury are calcium overload, formation of free oxygen radicals, reduced high-energy phosphate levels and abnormalities in microcirculatory flow [3, 8]. There is a substantial elevation in the level of cathecolamines during CPB, ninefold to 15fold increase in the plasma epinephrine and twofold to fivefold increase in the plasma norepinephrine [9]. The highest level of cathecolamines has been documented late in the course of CPB and while the patient is rewarmed with a return to normal level after discontinuation of CPB [9]. This cathecolamine level elevation can induce a reversible desensitization of the beta-adrenergic receptor [10]. Chronic sympathetic stimulation in patients with poor left ventricular function and poor peripheral perfusion leads to a depletion of the myocardial stores of norepinephrine, reduction in density and chronic desensitization of the beta-adrenergic receptors [11]. Should inotropic support be administered “prophilactically”? Despite myocardial dysfunction, some patients may not require inotropic support in order to separate successfully from CPB. Therefore, initiating inotropic support indiscriminately may expose these patients to the deleterious effects of inotropes. On the other hand, the prophylactic administration of inotropes at the end of CPB in patients with preexisting myocardial dysfunction, will make separation more likely to occur uneventfully with less variation in the systemic and pulmonary pressures and with an adequate cardiac output and peripheral perfusion pressure [12]. Stunned myocardium will respond to inotrope stimulation leading to improved contractile function and cardiac output. However, this is achieved at the expense of increased myocardial consumption and the combination of increased myocardial oxygen consumption and depleted energy reserves may cause irreversible damages [7]. Inotropes may adversely influence diastolic function with increased diastolic ventricular filling pressures, which lead to a decrease in the blood flow in the subendocardial vessels and an increase in the ventricular wall tension [12]. Some inotropes will induce a substantial increase in heart rate, which will result in a shortening of the diastolic coronary perfusion period and an increased oxygen demand. An elevated heart rate has also been associated with worsened outcome in patients with coronary artery disease [13]. In an attempt to identify patients with clinical need of inotropic support at the separation from CPB, numerous studies have looked at preoperative and intraoperative predictors. Some studies have identified factors such as increased age, low ejection fraction, female gender, cardiomegaly, history of congestive heart failure, repeat operation, emergency operation, recent myocardial infarction and left main coronary artery disease [14-16]. McKinlay et al in a study including patients undergoing either coronary artery bypass (CABG) or combined CABG and valve surgery and incorporating comprehensive transesophageal echocardiography (TEE) examination identified the following predictors: cross-clamp time, a greater number and/or severity of left ventricle wall motion abnormalities, left ventricular ejection fraction <35%, reoperation, combined CABG and mitral valve surgery and moderate or severe mitral regurgitation. Which inotrope or combination of inotropes is best? Inotropic drugs available for separation from CPB include phosphodiesterase inhibitors and beta-adrenergic agonists; commonly used are dopamine hydrochloride, dobutamine hydrochloride, milrinone and epinephrine. However, despite a wide range of available inotropic agents, no consensus exists regarding the treatment of LCOS post CPB [17] Epinephrine stimulates alpha- and beta- adrenergic receptors in dose dependent fashion with beta effects predominating at low doses and alpha effects predominating at high dose. Increased contractility is seen with all dosages, however systemic vascular resistance (SVR) may decrease, remain unchanged or increase substantially depending on the dosage. Epinephrine usually increases cardiac index (CI) but at increased dosages, predominant alpha-receptor mediated vasoconstriction may cause a decrease in the stroke volume (SV) because of an increase in afterload. Butterworth et al showed that during emergence from CPB, epinephrine (0.03mcg/kg/min) increased CI and SV by 14% without significant increase in heart rate (HR) [18]. Several studies have compared epinephrine with amrinone, milrinone and dobutamine. When compared with dobutamine, epinephrine was found to be less effective at increasing CI, however dobutamine was associated with significantly more tachycardia for the same stroke volume index increase [18, 19]. Epinephrine (0.03 mcg/kg per min) had no effect on left ventricular end-diastolic area (as an index of left ventricular compliance), measured using TEE, whereas milrinone significantly increased it by 15% [20]. Two studies have investigated the effect of epinephrine on the internal mammary artery graft flow by using laser Doppler flowmetry and have found that epinephrine has no effect [21, 22]. Dobutamine is a synthetic catecholamine, with strong affinity for beta1-adrenergic receptors and limited beta2 and alpha1 effects. On the heart, dobutamine increases inotropy via both beta1 and alpha1 effect and increases HR only through the beta1 effect. On the blood vessels, dobutamine is mainly a vasodilator, however in patients receiving beta-blockers, dobutamine may increase SVR predominantly through an alpha1 effect [23]. The European Milrinone Multicenter Trial Group in a randomized controlled trial comparing dobutamine with milrinone showed that dobutamine caused greater increases in CI and HR, but not stroke volume index (SVI), when compared with milrinone and greater increases in MAP and left ventricular stroke work index. Dobutamine was associated with a significantly higher incidence of hypertension and change from sinus rhythm to atrial fibrillation [24]. Romson et al showed that the dominant mechanism by which dobutamine improves left ventricular performance is by increasing HR and that low-dose dobutamine infusion affects left ventricular SVI differently as a function of the baseline SVI [25]. This study as well as a more recent study suggested that using prophylactic dobutamine infusion to separate from CPB irrespective of cardiac performance might actually be detrimental to some patients [25, 26]. Milrinone is a powerful cardiac inotrope and vasodilator, which increases intracellular c-AMP concentrations through inhibition of the c-AMP specific phosphodiesterase (PDE-III) in cardiac and smooth muscle cells. Increased c-AMP causes increased inotropy, lusitropy, chronotropy, dromotropy and increased automaticity. While thrombocytopenia has been a potential clinical concern with the administration of PDEIII inhibitors, George et al showed no significant reduction in the platelet count after 48 hours of milrinone infusion in cardiac surgical patients [27]. Kikura et al investigated the effects of milrinone on hemodynamics and LV function in cardiac surgical patients who were already treated with cathecolamines and found that milrinone administered as a loading dose only or as a loading dose followed by infusion significantly improved CI and the velocity of circumferential fiber shortening with no significant increase in HR and mean arterial pressure immediately after emergence from CPB when preload was maintained constant [28]. Milrinone has been compared with dobutamine in the European Milrinone Multicenter Trial; the significant findings have been presented in the previous section. Lobato et al showed that milrinone improved left ventricular compliance by increasing LV end-diastolic area by 15% as measured by TEE. However, in a later study, which focused on left ventricular relaxation post CPB as measured by color M-mode and tissue Doppler imaging, neither milrinone, nor epinephrine exhibited favorable lusitropic effects [29]. In a prospective randomized study, milrinone was shown to increase the blood flow through the grafted internal mammary artery by 24% [22]. Dopamine has agonist dose-dependent effects on alpha1, beta1, beta2 and dopaminergic receptors and indirect effects by releasing myocardial norepinephrine [23] with beta effects predominating at low dose and alpha effects predominating at high dose. Its most appealing feature is that at low doses (<5mcg/kg/min) dopamine has preferential dopaminergic and beta-adrenegic effects and increases renal perfusion promoting diuresis and natriuresis. Despite this action, a large, multicenter, randomized, controlled trial has shown that low-dose dopamine administered to critically ill patients who are at risk of renal failure does not confer clinically significant protection from renal dysfunction [30]. Studies have shown that although dopamine may increase CI, it does so at the expense of elevation in HR and adverse cardiac events (arrhythmias, ischemia, hypertension) [31, 32]. Enoximone is an imidazolone derivative PDE-III inhibitor. Tarr et al compared the hemodynamic effects of enoximone, dopamine and dobutamine in 75 patients undergoing mitral valve surgery in a prospective, randomized, blinded trial. They found that the patients in the enoximone group exhibited a significantly lesser increase in HR and a greater increase in stroke index than did either the dopamine or dobutamine group, and also exhibited a significantly greater increase in CI and decrease in SVR in comparison with dopamine [31, 32]. Levosimendan is a new positive inotropic drug, belonging to the class of calcium sensitizers. Levosimendan stabilizes the calcium-induced conformational change of cardiac troponin C (cTnC) only during systole by binding to the calcium-saturated conformation of cTnC, prolonging the systolic interaction of actin-myosin filaments without alteration of the cross-bridging cycling [33]. In contrast to other positive inotropic drugs, levosimendan does not increase intracellular calcium and avoids the undesired side effects of increased intracellular calcium such as increased oxygen consumption and increased risk for arrhythmia [33, 34]. At concentrations exceeding the clinically therapeutic concentrations for inducing positive inotropic effects, levosimendan is a highly selective inhibitor of phophodiesterase III. However, at pharmacologically relevant concentrations of 0.03–0.1 _M, levosimendan does not alter heart rate, cAMP levels, myocardial relaxation, and cytosolic calcium [33]. There is little published work on the use of levosimendan in cardiac surgical patients with LCOS. The LIDO study, a randomized, double-blinded, multicenter trial, compared the effects of levosimendan with dobutamine in 203 patients with acute decompensated low-output heart failure. The study showed that levosimendan improved hemodynamic performance more effectively than dobutamine. This benefit was accompanied by lower mortality in the levosimendan group than in the dobutamine group for up to 180 days [35]. Overall frequency of adverse events was similar in the two groups, however, headache tended to be associated more frequently with levosimendan, while rhythm disorders and myocardial ischemia were more common in the dobutamine group. Conclusion Inotrope prescribing habits vary by physician and center. There is no ideal inotrope at present. The use of inotropes at separation from CPB should be based on hemodynamic and TEE data and individualized to each case. 1. 2. Goldstein, D.J. and M.C. Oz, Mechanical support for postcardiotomy cardiogenic shock. Semin Thorac Cardiovasc Surg, 2000. 12(3): p. 220-8. Breisblatt, W.M., et al., Acute myocardial dysfunction and recovery: a common occurrence after coronary bypass surgery. J Am Coll Cardiol, 1990. 15(6): p. 1261-9. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. Royster, R.L., Myocardial dysfunction following cardiopulmonary bypass: recovery patterns, predictors of inotropic need, theoretical concepts of inotropic administration. J Cardiothorac Vasc Anesth, 1993. 7(4 Suppl 2): p. 19-25. Reichert, C.L., et al., Prognostic value of biventricular function in hypotensive patients after cardiac surgery as assessed by transesophageal echocardiography. J Cardiothorac Vasc Anesth, 1992. 6(4): p. 429-32. Furukawa, S., et al., Relationship between total mechanical energy and oxygen consumption in the stunned myocardium. Ann Thorac Surg, 1990. 49(4): p. 543-8; discussion 548-9. Kusuoka, H., et al., Excitation-contraction coupling in postischemic myocardium. Does failure of activator Ca2+ transients underlie stunning? Circ Res, 1990. 66(5): p. 1268-76. Westaby, S., L. Balacumaraswami, and R. Sayeed, Maximizing survival potential in very high risk cardiac surgery. Heart Fail Clin, 2007. 3(2): p. 159-80. Opie, L.H., Reperfusion injury and its pharmacologic modification. Circulation, 1989. 80(4): p. 1049-62. Reves, J.G., et al., Neuronal and adrenomedullary catecholamine release in response to cardiopulmonary bypass in man. Circulation, 1982. 66(1): p. 49-55. Schwinn, D.A., et al., Desensitization of myocardial beta-adrenergic receptors during cardiopulmonary bypass. Evidence for early uncoupling and late downregulation. Circulation, 1991. 84(6): p. 2559-67. Bristow, M.R., et al., Decreased catecholamine sensitivity and beta-adrenergicreceptor density in failing human hearts. N Engl J Med, 1982. 307(4): p. 205-11. Hardy, J.F. and S. Belisle, Inotropic support of the heart that fails to successfully wean from cardiopulmonary bypass: the Montreal Heart Institute experience. J Cardiothorac Vasc Anesth, 1993. 7(4 Suppl 2): p. 33-9. Hjalmarson, A., et al., Influence of heart rate on mortality after acute myocardial infarction. Am J Cardiol, 1990. 65(9): p. 547-53. Butterworth, J.F.t., et al., Factors that predict the use of positive inotropic drug support after cardiac valve surgery. Anesth Analg, 1998. 86(3): p. 461-7. Rao, V., et al., Predictors of low cardiac output syndrome after coronary artery bypass. J Thorac Cardiovasc Surg, 1996. 112(1): p. 38-51. Royster, R.L., et al., Preoperative and intraoperative predictors of inotropic support and long-term outcome in patients having coronary artery bypass grafting. Anesth Analg, 1991. 72(6): p. 729-36. Gillies, M., et al., Bench-to-bedside review: Inotropic drug therapy after adult cardiac surgery -- a systematic literature review. Crit Care, 2005. 9(3): p. 266-79. Butterworth, J.F.t., et al., Dobutamine increases heart rate more than epinephrine in patients recovering from aortocoronary bypass surgery. J Cardiothorac Vasc Anesth, 1992. 6(5): p. 535-41. Balderman, S.C. and J. Aldridge, Pharmacologic support of the myocardium following aortocoronary bypass surgery: a comparative study. J Clin Pharmacol, 1986. 26(3): p. 175-83. Lobato, E.B., N. Gravenstein, and T.D. Martin, Milrinone, not epinephrine, improves left ventricular compliance after cardiopulmonary bypass. J Cardiothorac Vasc Anesth, 2000. 14(4): p. 374-7. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. Cracowski, J.L., et al., Effect of low-dose positive inotropic drugs on human internal mammary artery flow. Ann Thorac Surg, 1997. 64(6): p. 1742-6. Lobato, E.B., et al., Effects of milrinone versus epinephrine on grafted internal mammary artery flow after cardiopulmonary bypass. J Cardiothorac Vasc Anesth, 2000. 14(1): p. 9-11. Balser, J.R.B., J.F. ; Larach D.R., Cardiovascular Drugs. 4th ed. A practical approach to cardiac anesthesia, ed. F.A.M. Hensley, D.E; Garvlee, G.P. 2008, Philadelphia: Lippincott Williams & Wilkins. Feneck, R.O., et al., Comparison of the hemodynamic effects of milrinone with dobutamine in patients after cardiac surgery. J Cardiothorac Vasc Anesth, 2001. 15(3): p. 306-15. Romson, J.L., et al., Effects of dobutamine on hemodynamics and left ventricular performance after cardiopulmonary bypass in cardiac surgical patients. Anesthesiology, 1999. 91(5): p. 1318-28. Fellahi, J.L., et al., Perioperative use of dobutamine in cardiac surgery and adverse cardiac outcome: propensity-adjusted analyses. Anesthesiology, 2008. 108(6): p. 979-87. George, M., J.J. Lehot, and S. Estanove, Haemodynamic and biological effects of intravenous milrinone in patients with a low cardiac output syndrome following cardiac surgery: multicentre study. Eur J Anaesthesiol Suppl, 1992. 5: p. 31-4. Kikura, M., et al., The effect of milrinone on hemodynamics and left ventricular function after emergence from cardiopulmonary bypass. Anesth Analg, 1997. 85(1): p. 16-22. Lobato, E.B., et al., Effects of milrinone versus epinephrine on left ventricular relaxation after cardiopulmonary bypass following myocardial revascularization: assessment by color m-mode and tissue Doppler. J Cardiothorac Vasc Anesth, 2005. 19(3): p. 334-9. Bellomo, R., et al., Low-dose dopamine in patients with early renal dysfunction: a placebo-controlled randomised trial. Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Lancet, 2000. 356(9248): p. 2139-43. Rosseel, P.M., et al., Postcardiac surgery low cardiac output syndrome: dopexamine or dopamine? Intensive Care Med, 1997. 23(9): p. 962-8. Tarr, T.J., et al., Haemodynamic effects and comparison of enoximone, dobutamine and dopamine following mitral valve surgery. Eur J Anaesthesiol Suppl, 1993. 8: p. 15-24. Toller, W.G. and C. Stranz, Levosimendan, a new inotropic and vasodilator agent. Anesthesiology, 2006. 104(3): p. 556-69. Lehmann, A. and J. Boldt, New pharmacologic approaches for the perioperative treatment of ischemic cardiogenic shock. J Cardiothorac Vasc Anesth, 2005. 19(1): p. 97-108. Follath, F., et al., Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet, 2002. 360(9328): p. 196-202.