* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
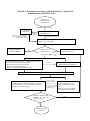
Download Strategy Plan for Execution of Influenza Pandemic Response
Eradication of infectious diseases wikipedia , lookup
Compartmental models in epidemiology wikipedia , lookup
Public health genomics wikipedia , lookup
Marburg virus disease wikipedia , lookup
Viral phylodynamics wikipedia , lookup
Henipavirus wikipedia , lookup
Swine influenza wikipedia , lookup
Transmission (medicine) wikipedia , lookup
Human mortality from H5N1 wikipedia , lookup
Influenza A virus subtype H5N1 wikipedia , lookup
Infection control wikipedia , lookup
Strategy Plan for Execution of Influenza Pandemic Response Department of Health, Executive Yuan Republic of China (Taiwan) January 2007 Table of Contents Foreword 8 1. Background 11 1.1 Current Epidemic Situation 11 1.2 Administrative Mechanisms 15 1.2.1 Central Government Mechanisms 15 1.2.1.1 National Security Level 15 1.2.1.2 Executive Yuan Level 16 1.2.1.3 Department of Health Level 16 1.2.1.4 Emergency Mobilization Mechanisms 16 1.2.2 Local Government Mechanisms 17 1.2.3 Exercises 18 1.3 Outline of Strategies 19 1.3.1 Four Major Strategies 19 1.3.2 Five Lines of Defense 21 1.4 Classification of the Pandemic Situation 23 1.4.1 WHO Classification of Pandemic Phases 23 1.4.2 Taiwan Classification of Pandemic Phases 25 1.4.3 Comparison of Classification of Pandemic Phases between Our 26 Country and WHO 2. Surveillance 27 2.1 Rationale 27 2.2 Implementation Strategies 29 -1- 2.2.1 Usage of Multiple Surveillance Systems 29 2.2.1.1 Current Surveillance Systems 29 2.2.1.2 Operation of Surveillance Systems 30 2.2.2 Laboratory Diagnosis 31 2.2.2.1 Techniques of Laboratory Diagnosis 31 2.2.2.2 Execution of Laboratory Diagnosis 32 2.2.3 Case Investigation 32 2.2.3.1 Execution of Case Investigation 33 2.2.3.2 Management of Close Contacts 33 3. Antivirals Strategy 35 3.1 Rationale 35 3.2 Implementation Strategies 36 3.2.1 Stockpile Quantity of Antivirals 36 3.2.2 Time of Administration 36 3.2.3 Administration Plan of Antivirals in the Future 37 3.2.4 Procedures of the Use of Tamiflu API 38 3.2.4.1 Activation Mechanism for Use 38 3.2.4.2 Packaging and Labeling 39 3.2.4.3 Medicine Prescription 39 3.2.4.4 Delivery 40 3.2.5 Information Management 40 3.2.6 Activation of Domestic Manufacture of Tamiflu 41 4. Vaccine Strategy 43 4.1 Rationale 43 -2- 4.2 Implementation Strategies 45 4.2.1 Short-Term: Stockpile of “Pandemic-like”Vaccine 45 4.2.2 Medium-Term: Self-manufacturing of Emergency Vaccine 45 4.2.3 Long-Term: Plan for Domestic Vaccine Manufacture 48 4.2.4 Establishment of Capability in Virus Strain Selection 49 4.2.5 Administration of Pandemic Vaccine 49 5. Transmission Interruption Measures 50 5.1 Rationale 50 5.2 Implementation Strategies 52 5.2.1 Infection Control Strategies 53 5.2.2 Contact Restriction Strategies 55 5.2.2.1 Isolation 55 5.2.2.2 Quarantine 56 5.2.2.3 Community restriction 58 5.2.2.4 Sheltering 59 6. Preparedness of Personal Protective Materials 61 6.1 Rationale 61 6.2 Implementation Strategies 62 6.2.1 Estimation of PPE Demand 62 6.2.2 Determination of Safety Stockpile 62 6.2.3 Information Management and Monitoring 63 6.2.3.1 Real-time of Information 64 6.2.3.2 Stockpile Inspection and monitoring for Abnormality 64 Management -3- 6.2.4 Reallocation and Delivery 65 6.2.4.1 Reallocation 65 6.2.4.2 Delivery 65 6.2.5 Dealing with PPE Shortage 66 6.2.5.1 Reduce Expenditure 66 6.2.5.2 Speed up Supply 66 6.2.5.3 Stockpile and Release of Masks for Common Need 67 6.3 Emergency Handling of Abnormality 68 6.3.1 Back-Up Personnel of PPE Reallocation 68 6.3.2 Emergency Contact Points 68 7. Maintaining Health Services 70 7.1 Rationale 70 7.2 Implementation Strategies 71 7.2.1 Primary Care Settings 71 7.2.1.1 Home Treatment 73 7.2.2 Medical Network for Prevention and Control of Infectious 74 Diseases 7.2.3 Large Care Facilities 75 7.2.4 Handling of Off-Shore Patients 76 7.2.5 Deployment of Medical Staffs 77 7.2.6 Compensation for Personnel Deployment and Requisition 78 8. Response and Execution 79 8.1 Phase O+ phase3 79 8.2 Phase A1+ phase4 80 -4- 8.3 Phase A1+ phase5 82 8.4 Phase A2+ phase3~5 84 8.5 Phase B+ phase5 86 8.6 Phase C+ phase6 89 9. Risk Communication 91 9.1 Correct Consumption of Poultry Products 91 9.1.1 Rationale 91 9.1.2 Implementation Strategies 91 9.2 Strengthening of Respiratory Hygiene/Cough Etiquette 93 9.2.1 Rationale 93 9.2.2 Implementation Strategies 93 9.3 Correct Usage of Respirators and Medical Masks 94 9.3.1 Rationale 94 9.3.2 Implementation Strategies 95 9.3.2.1 Usage of Medical Masks 95 9.3.2.2 Usage of N95 or Higher Level Masks 96 9.3.2.3 Selection of Masks 97 9.3.2.4 Clarification of Related Concepts 98 9.3.2.4.1 Transmission Routes of Influenza Virus 98 9.3.2.4.2 Medical Mask Is Not a Respiratory Protection Gear 100 9.3.2.4.3 Recommendations on Using Masks for Common People 101 9.3.2.4.4 Materials of Mask and Filtration Mechanisms of Airborne 105 Particles 9.4 Public Seeking Medical Help 106 -5- 9.4.1 Rationale 106 9.4.2 Implementation Strategies 108 9.5 Business and Organization Continuity 117 9.5.1 Rationale 117 9.5.2 Implementation Strategies 117 10. Exercises 120 10.1 Rationale 121 10.1.1 Purpose of Exercise 121 10.1.2 Exercise Types 121 10.1.3 How to Plan An Exercise 123 10.1.4 Organizational Structure of Exercise 125 10.1.5 Affairs to be Managed After Exercise 127 10.2 Implementation Strategies 127 10.2.1 International Exercise 128 10.2.2 Central-Level Exercise 130 10.2.3 Local-Level Exercise 134 Appendix 1. Characteristics of Avian Influenza A (H5N1) virus 137 Appendix 2. Estimation on Health Impact of Influenza Pandemic 144 Appendix 3. Excerpt of Presidential Instructions to a High Level National 147 Security Meeting Appendix 4. Enforcement Regulations Governing the Central Epidemics 156 Command Center Appendix 5. Exercises in Response to Influenza Pandemic 170 Reference 173 -6- Table of Contents Chart Hierarchy of related plans in response to influenza pandemic 10 Chart 6.1 Safety Stockpile of PPE 63 Chart 9.1 Planned Procedures of Communication With Patients Seeking 112 Medical Help — Out-Patient visits/Hospitalization Chart 9.2 2006 ” Medical Network for Prevention and Control of Infectious 114 Diseases” Flowchart of Activation of Infectious Disease Prevention and Control Hospitals Chart 9.3 “Medical Network for Prevention and Control of Infectious 115 Diseases” Procedure Flowchart for Transfer of Infected Patients from Off-Shore Area to Main Island for Treatment Chart 9.4 “Patient doesn’t move, physicians moves” Operation Procedures 116 for off-shore Area Table 3.1 Time of Administration of Antivirals 42 Table 6.1 PPE Stockpile and Allocation 63 Table 9.1 Comparison of Product Specifications between Respirator and 101 Medical Mask Table 9.2 Comparison of Functions of Respiratory protection Gear and Masks -7- 104 Foreword Influenza is the most potential pandemic disease. Since 2004, the World Health Organization (WHO) has continued to announce cases of Influenza A virus subtype H5N1 in human. Various global information show that influenza pandemic is approaching. And all developed countries are preparing for its coming. Influenza pandemic affects not only our people’s life and health, it will also lead to pressure and disorder both socially and economically. The national goals of the influenza pandemic response are as follows: 1. Preclude occurrence – Preclude the domestic occurrence of any single human H5N1 case before pandemic outbreak 2. Avoid transmission – Once H5N1 virus has been imported from abroad, every effort will be implemented to prevent further domestic transmission 3. Reduce harm – If H5N1 virus becomes more contagious, aggressive intervention of medical and public health means will be implemented to reduce its impact to citizen’s health. Social functions and economic activities will keep functioning. 4. Effective recovery – After the pandemic period, social psychological and economic recovery plans will be launched. In order to be prepared, the Executive Yuan has ratified “National Influenza Pandemic Preparedness Plan” (“Preparedness Plan” in short) in May 2005. All levels of government should proceed with preparedness according to the plan, such as stocking medical and epidemic prevention materials, establishment of mobilization structure, training of epidemic prevention manpower, etc. This “Strategy Plan for Execution of Influenza Pandemic Response”(“Strategy Plan” in short) is in response to the strategies and guidelines that WHO announced in succession. Recent prevention concepts of -8- other developed countries were also referred to. Possible epidemic prevention measures by government during different epidemic phases were studied. Rationale and principle of each measure are described logically. All government organizations and people can get a complete picture of the strategy, and detailed epidemic prevention plans can be pre-drafted by various authorities according to their respective responsibilities. Centers for Disease Control (CDC) of Department of Health of Executive Yuan published the “Mobilization and Preparedness Plan for Influenza Pandemic Prevention (Implementation Plan)” (“Battle Plan” in short) in February, 2005. The Battle Plan is based on our country’s previous epidemic control experience. Standard operation procedures and the division of responsibilities among government authorities have been established in the areas of epidemic surveillance, onsite health management of domestic poultry outbreaks, medical care of patients, case management, and material usage and control, so that related authorities can cooperate in execution of tasks. The Battle Plan is based on the SOP defined in Strategy Plan, but it will be constantly updated and announced according to the latest situation. -9- Chart. Hierarchy of related plans in response to influenza pandemic Corresponding WHO documents Project Name National Influenza Avian influenza: assessing the pandemic threat. Jan. 2005. WHO global influenza preparedness plan. ---- Mar. 2005. Pandemic Preparedness Plan Hierarchy illustration The highest guiding principles ratified by the Executive Yuan ----- Striving for budgets accordingly for various preparedness tasks (Preparedness Plan) outlined ↓ ---- WHO checklist for influenza pandemic Influenza Pandemic ----- Outline of Principles for various control measures Response preparedness planning. 2005. Execution strategy set up according to “Preparedness Plan” Execution of pandemic threat: Recommended strategic actions. Sep. 2005. Strategy Plan for Responding to the avian influenza (Strategy Plan) Basis of every organization in devising actual measures ↓ WHO guidelines for global surveillance of influenza A/H5. Jan. 2004. WHO pandemic influenza draft protocol for rapid response and containment. May. 2006. Avian influenza, including influenza A (H5N1) in humans: WHO interim infection control guideline for health care facilities. Apr. 2006. WHO rapid advice guidelines on pharmacological management of humans ---- for Influenza Pandemic Prevention (Battle Plan) virus. May. 2006. A Manual for improving bio-security in the food supply Chain: Focus on live animal markets. Apr. 2005. - 10 - SOP established according to “Strategy Plan” Preparedness Plan infected with avian influenza A(H5N1) Mobilization and ----- Details of execution of epidemic prevention by each unit Will be constantly updated according to latest situation 1. Background 1.1. Current Epidemic Situation A possible threat of influenza pandemic comes from the epidemic spread within fowls caused by influenza A virus subtype H5N1 and the occurrence of human cases. Since the middle of year 2003, highly pathogenic avian influenza (HPAI) has ravaged poultry and animal farms in 8 Southeast Asia countries. The pathogen was influenza A subtype H5N1 avian flu. This virus existed in these countries since then, and is hard to eradicate. Again between July and the end of year 2005, the geographic distribution of fowl infections by H5N1 virus extended beyond Asia. Cases of wild and domestic birds infected by H5N1 virus were reported in Russia, Kazakhstan, Turkey, Romania and Ukraine. Wild bird infections were reported in Croatia and Mongolia. The widespread was even more extensive during February and April 2006. There were 32 countries in Africa, Asia, Europe and Middle East reporting their first H5N1 infection case in wild or domestic bird. Some of these affected areas are highly populated. Some lack of surveillance and medical care system. Therefore the risk of human infection increases. According to research, the virus of avian flu spread from Russia to Kazakhstan, Nigeria, Iraq, and Turkey and into Europe can be traced back to the same source with the avian flu virus that infected wild birds in Qinghai Lake in northwestern China in spring 2005.[1,2] Besides, a survey around Poyang Lake in China showed that as much as 3.1% of wild duck excretion was H5N1 antibody positive, which shows that the virus is adaptive in certain wild fowls and is able to spread along the migratory path of migrant birds. The researches confirmed the important role of migratory birds in H5N1 virus spread and its possibility to accelerate the global widespread of avian flu. Other scholars suggested that, besides the migration of migratory birds, human trading and smuggling conduct cannot be taken lightly. Even though - 11 - several leading countries in poultry husbandry have prohibited the export of related agricultural product after their HPAI outbreak, virus might have already spread into import countries quietly before the prohibition. And smuggling will be even more difficult to anticipate and prevent. Statistics show that among all smuggled goods, the ranking of fowls and its related products is the third highest in quantity, only next to narcotics and firearms. With import prohibition announced by certain countries, the risk of smuggling fowls and its related product becomes higher. And it will be harder to block the transmission and spread of avian flu virus. Because the existence scope of the virus expands, human exposure to the virus is getting higher and the risk of infection increases. With every one case of human H5N1 avian flu, the virus becomes more adaptive to human body, or the risk of gene reassortment of human and fowl virus increases, hence forming a new human influenza virus. Once this virus evolves and can easily spread among humans, a majority of the world population will be infected within a short period of time, because no one carries an antibody against the new virus. This is the so-called “influenza pandemic”. As for the human cases in H5N1, the first confirmed case of this epidemic wave was found in Vietnam in December 2003. Until January 9, 2007, there were 263 confirmed cases. Reporting countries include Azerbaijan, Cambodia, China, Djibouti, Egypt, Indonesia, Iraq, Thailand, Turkey and Vietnam. H5N1 virus caused serious systemic disease in these patients. Among these patients, 157 have died. Most patients were children and young adults, with unknown reasons. WHO announced on 30 July, 2006 the epidemiologic information of the 205 human H5N1 cases collected until April, 2006. Analysis showed that about 50% of the cases are children or adolescent under 20 years old. The median of the time between falling ill and hospitalization is 4 days. Mortality rate of all - 12 - cases is 56%. Mortality rate in 10-19 year old cases is as high as 73%. Among death cases, the median of the time between falling ill and death is 9 days. However, the percentage of infection without symptom or with minor symptoms is unknown, and further research is necessary. Even though cases were reported all year, more cases were reported in colder seasons. If this infection pattern persists, another peak of infection is expected to come from the end of 2006 to the beginning of 2007[4]. According to recent evidence, the species barrier of H5N1 virus still exists. In other words, this virus is still fowl adaptive, and it’s not easy to be transmitted from fowl to human. After investigation of human cases, it is found that they mostly inhabited in HAPI-affected areas and had engaged in risky activities which involved close contact with ill or dead fowls, such as butchery, defeathering or cooking. There was one possible limited human-to-human transmission event in Thailand in year 2004. Three patients were infected. The index patient’s last contact with home-bred ill chicken was three to four days before falling ill. The index patient’s mother came home from another city to take care of the patient in hospital, and later died of pneumonia. The index patient’s aunt was also infected. Neither of them had appropriate protection while taking care of the index patient. In Indonesia, there was also a household cluster of human H5N1 cases in May 2006, which constituted another of the very few events of possible limited human-to-human transmission. After WHO experts’ investigation, it was found that virus didn’t spread within the community. No health care worker was infected either. It showed that infection is only possible with very close contact with H5N1 infected patients. Therefore the global pandemic alert phase is still maintained at level 3. That is to say, even though there were occasional human cases of H5N1 virus infection, no evidence supported that the virus can be transmitted among people effectively and continuously. - 13 - In the aspects of social and economic influences caused by influenza pandemic, it is estimated that the possible number of death will be approximately between 2 and 7.4 millions if pandemic occurs now, calculating according to influenza pandemic pattern in year 1968. If the virulence of this pandemic virus is as strong as the one in 1918, then the number of deaths will be far more than this. Besides, in the economical aspect, tourism, mass transportation, retail sale, food and beverage and manufacturing industry will all be influenced. According to the experience of SARS period, the GDP of East Asia countries dropped 2% in the 2nd quarter of 2003 while SARS ravaged the region. The number of deaths at that time was 800 people. Ministry of Economic Affairs estimates that Taiwan’s GDP will drop 2.85% if the next pandemic lasts three months. Assuming GDP of all countries drops 2% and the epidemic situation persists for one year, there will be a pecuniary loss of US$ 800 billion. For the characteristics of Avian Flu virus A subtype H5N1, please refer to Appendix 1. For the health impact of influenza pandemic, please refer to Appendix 2. - 14 - 1.2 Administrative Mechanisms 1.2.1 Central Government Mechanisms The periodical Foreign Affairs, which always reports international politic issues, published with huge capacity to comment on the possibility of the attack of influenza pandemic[5]. In the same month, our country also defined the possible pandemic caused by H5N1 virus to be a “non-traditional” threat to national security. In May 2005, APEC Ministerial Meeting on Avian and Influenza Pandemics was held in Vietnam[6]. Influenza pandemic, tsunami, earthquake, man-made disastrous event and space garbage were defined as principal events of dealing with global emergency. Influenza pandemic was positioned in preventive phase. Other events were positioned in preparatory phase, responsive phase and recovery phase respectively. Therefore the administration level of Influenza pandemic control has been raised to National security level. A three-tier control and management hierarchy has been established at the levels of Presidential Office (National Security level), Executive Yuan and Department of Health (DOH). 1.2.1.1 National Security Level The “National Security Meeting ” in response to avian and influenza pandemic chaired by the President analyses national and international epidemic situations, and based on its evaluation of the possible impact of a pandemic to national security, strategically instructions are given to Executive Yuan and related departments. There have been three meetings from August 2005 to March 2006. Please refer to Appendix 3 for detailed instructions by the President in the three National Security Meetings. - 15 - 1.2.1.2 Executive Yuan Level Since 25 October 2005, Executive Yuan has held the “Executive Yuan Coordination Meeting for Avian Influenza Prevention and Control” (“Coordination Meeting” in short) periodically. Premier of Executive Yuan has assigned a convener. When the domestic epidemic situation level is O, Council of Agricultural of Executive Yuan assists the convener. Once domestic fowls have tested positive for H5 or H7 HPAI, Department of Health of Executive Yuan (DOH) will assist. Every related department is assembled to discuss, debate and make strategic decisions on each cross-sectoral or important issue related to avian flu and pandemic flu. Up to December 2006, there have been 22 such meetings. In addition, in response to national and international epidemic situations, unscheduled reporting will be given in Executive Yuan meetings. 1.2.1.3 Department of Health Level Since September 2005, DOH has held the “Preparedness and Mobilization Meeting for Influenza Pandemic Control” (“Preparedness Meeting” in Short). This meeting discusses preparedness work for a possible epidemic and every preventive measure currently underway is reviewed according to respective WHO guidelines. Important issues to be brought up to the above mentioned Coordination Meeting for discussion or affairs handed-down from Coordination Meeting to be implemented and followed up will be covered in the agenda of the Preparedness Meeting. Up to December 2006, there have been 49 such meetings. 1.2.1.4 Emergency Mobilization Mechanisms We are currently in phase 0. CDC analyzes international epidemic situations to grasp their development, and reports to higher authorities at any - 16 - time. The occurrence of influenza pandemic is bound to be an emergent and serious event, and no single government organization is capable of complete control. Therefore, once the situation worsens, Executive Yuan will establish a Central Epidemic Command Center for Influenza Pandemic Level A1~C according to “Communicable Disease Control Act” and “Enforcement regulations Governing the Central Epidemics Command Center”. The center will be run under the spirit and framework of Incidence Command System (ICS). A single command system will facilitate efficient coordination of resources, equipment and staffs. Regional Command Centers will be established according to the administrative jurisdictions of CDC branches to facilitate regional joint defense and smooth flow of orders and information. Please refer to Appendix 4 for Enforcement Regulations Governing the Central Epidemics Command Center. 1.2.2 Local Government Mechanisms Article 4 of the Communicable Disease Control Act defines the division of responsibilities among central and local (special municipality, city and county) government organizations in implementing infectious disease prevention and control measures. It is also applicable to the control of influenza pandemic. Central government devises policy and plans for prevention and control. Local government should draft local response and implementation plans according to local needs and put them into practice. Central organization should make regular and irregular assessment of these plans. To implement the preparedness and response measures for influenza pandemic, local government should draft a local implementation plan and prepare for the required budget according to the preparedness plan, strategy plan and battle plan. Other than that, a cross-sectoral mechanism should be properly established to manage and make use of the area’s medical resources, to store - 17 - necessary epidemic preventive materials, and to examine hospital infection control. Every type of non-governmental organizations and voluntary groups that could be mobilized in the community should be duly noted to facilitate future mobilization of related resources for epidemic control once an epidemic command center is established based on Item 3 of Article 4 of the Communicable Disease Control Act. In the future, the Central Epidemics Command Center may decide to implement strategies such as rapid containment (including measures such as restriction of movement in and out of the area, ”ring” prophylaxis, restriction on social contact, etc) and establishing large-scale care facilities. Local government should plan in advance the operation mode, and mobilize various human and material resources timely to prevent discontent of the people and effectively control the epidemic situation. When the Central Epidemics Command Center for Level A1 is established, a local government can decided not to establish a county/city command center after evaluating the extent of internationalization and risk of epidemic situation in its jurisdiction. However, contact points must be assigned to enable smooth flow of information and commends. When the epidemic level upgrades to A2, all county/city governments should establish a local command center and active the response mechanism whether infected cases are present in their jurisdictions or not. 1.2.3 Exercises Central government has conducted 13 exercises in total from July 2005 to October 2006. Six of them were hosted by the DOH, including 3 tabletop exercises simulating the operation of the Central Epidemics Commend Center, and 4 drills for specific preventive strategies (incoming passengers at the airport, prescription of antiviral agents, PPE emergency distribution, medical team’s - 18 - deployment to off-shore islands).The Council of Agriculture also conducted drills on culling work for HPAI. Local governments held 40 drills in total from January to October, 2006 with all county/city governments having been involved. The modes included tabletop exercises and drills, and the scenarios involved evacuation of hospitals, requisition of non-infectious disease prevention hospitals, response actions of market retailers and street vendors, patient managements and transfer disease importation through mini-three link with China, ad-hoc diagnosis/treatment centers, etc. Appendix 5 contains a detailed list of all exercises and drills. For detailed principles and strategies of exercises, please refer to Chapter 10 of this Strategy Plan. All exercises and drills concerning influenza pandemic are coded “Egret” for identification after August 2006. 1.3 Outline of Strategies In response to influenza pandemic, our country set up “4 major strategies and 5 lines of defense” as the main framework for pandemic control, in order to provide sufficient health protection to our countrymen. 1.3.1 Four Major Strategies WHO had included surveillance for pandemic preparedness, public health interventions, the use and availability of antivirals, and better access to better vaccines as four major topics for discussion in “WHO consultation on priority public health interventions before and during an influenza pandemic” held in March 2004[7]. The 4 major strategies in response to influenza pandemic set up by our - 19 - country also focus on early detection, interruption of transmission, anti-virals and influenza vaccine. Strategy I - Early detection The important function of surveillance is to detect unusual cluster of cases at an early stage or to discover abnormal clinical manifestations in cases and then to understand virus characteristics through analysis. This will help us to block the virus in time once its transmission ability enhances and will facilitate the execution of epidemic control measures to prevent the epidemic situation from worsening. Strategy II – Interruption of transmission Other than medical interventions such as anti-virals and vaccine, there are non-medical public health interventions such as personal hygiene practices (including washing hands frequently and wearing a mask when being ill), isolation of patients, control of contacts, social distancing, etc. All of them are important and economic preventive measures. Strategy III - Antivirals At present, the cure and preventive function of neuraminidase inhibitor anti-viral has been confirmed in seasonal influenza. As a consequence, it is expected to be effective in treatment and after exposure prophylaxis for avian influenza and pandemic influenza, so as to block the virus transmission or to reduce morbidity and mortality. Strategy IV - Influenza vaccine Annual influenza vaccination program has effectively decreased morbidity and mortality of seasonal influenza. Similarly, it is expected that, during influenza pandemic, sufficient amount of effective vaccine obtained through purchase or domestic manufacture to maintain major social function, even to safeguard the health of high-risk groups. - 20 - 1.3.2 Five Lines of Defense In response to influenza pandemic, our country set up 5 lines of defense including containment aboard, border quarantine, community epidemic control, maintaining normal functioning of medical system, and individual and family protection. Line of Defense I - Containment aboard At present stage, “Containment aboard” is the main objective. Only containment of the spread of virus at the early phase of its adaptation to human can we interrupt or delay the occurrence of influenza pandemic. Hence we can strive for more time to proceed with other preparedness. Therefore, it is essential in current stage that we actively participate in global collaboration plan in prevention and treatment, reinforce exchange and sharing of epidemic control information, and build up tight cooperation channels. At the same time, we should keep changes in the international epidemic situation under surveillance, and upgrade border control measures according to situation to prevent epidemic spread into our country. Line of Defense II - Border quarantine The risk of influenza pandemic occurring overseas is higher than domestically. Should the transmission ability of virus continues to increase, reinforcement of quarantine inspection in airports and seaports is a major method to protect our people’s health. Health monitoring and management of incoming passengers will be upgraded gradually depending on the international epidemic situation. Potential patients can be identified immediately and treated promptly to prevent epidemic spread within our country. Line of Defense III - Community epidemic control In the future, if transmission ability of influenza virus becomes extremely strong, and the virus is impossible to be blocked by containment aboard or - 21 - border quarantine measures which enable its spread into communities in our country, then community epidemic control will become the major method to decrease its impact. Furthermore, during influenza pandemic, supply of vaccine for pandemic virus strain may not be sufficient and timely and supply of anti-virals may also be limited, and hence non-medical interventions are absolutely indispensable. Although some of the non-medical interventions may influence people’s behaviors and rights, according to the Communicable Disease Control Act, the government is authorized to implement related epidemic control measures when an infectious disease occurred. However, to implement each public health intervention thoroughly, enforcement by the government is not enough. The key to diminish the spread of virus in community depends on whether people can understand the meaning of each epidemic control measure and thereupon to obey and cooperate. In the future, the government will combine forces with civil groups and volunteers to provide people with correct protection information, and to strengthen the public’s level of cooperation with community epidemic control measures. At present, DOH has started to recruit and to train backup manpower to deal with epidemic control requirements during influenza pandemic. Line of Defense IV - Maintaining normal functioning of medical system The attack rate of the 1918-1919 influenza pandemic was estimated to be 25%. Nowadays with frequent social interaction and convenient traffic, the attack rate is bound to increase should another influenza pandemic occur. When the time comes, a large number of influenza patients will definitely bring enormous challenge to the medical system. In order to prevent patients of other diseases from being deprived of medical resources during a pandemic, and to provide more extensive care to a large number of patients affected by the epidemic disease, CDC has established a National Medical Network for Prevention and Control of Infectious Diseases. It will provide emergency - 22 - response to national epidemic control needs. Besides, local governments should plan in advance the necessity of setting up large-scale care facilities should the capacity of Medical Network for Prevention and Control of Infectious Diseases was exceeded by patient number of pandemic influenza. Line of Defense V - Individual and family protection For prevention of most infectious diseases, it may be said that hygiene habits are the most basic factor. Correct hygiene habits should be formed in ordinary days and should maintained during pandemic period. In case there is influenza pandemic, people should stay home as much as they can to reduce unnecessary social interaction. In addition, people with mild disease may also need to recuperate at home. At this time, the government will demand higher levels of personal and household hygiene to be practiced but will strive to avoid causing public panic. 1.4 Classification of the Pandemic Situation 1.4.1 WHO Classification of Pandemic Phases [8] Warning status Phase Human risk situation Phase 1 Low risk of human cases Phase 2 High risk of human cases Phase 3 No or very limited human-to-human transmission Phase 4 Evidence of increased human-to-human transmission Phase 5 Evidence of significant human-to-human transmission Phase 6 Efficient and sustained human-to-human transmission Inter-pandemic Pandemic alert Pandemic Above table is the classification for warning of influenza pandemic alert in - 23 - the WHO Global Influenza Preparedness Plan announced in 2005. The purpose of classification is to provide appropriate suggestions for epidemic control preparation. In this WHO plan, different suggestions were also provided according to whether an individual country is affected by the new virus. Descriptions of each classification are as follow: Phase 1. No new influenza virus subtypes have been detected in humans. Low risk of human infection. Phase 2. No new influenza virus subtypes have been detected in humans, but an animal variant threatens human disease. Phase 3. Human infection(s) with a new subtype but no human-to-human spread, or only few cases of close contact spread. Phase 4. Small cluster(s) with limited localized human-to-human transmission. It is shown that virus is not complete adaptive to human bodies. In this phase, it is still possible to block or delay the spread of virus. Possible scenarios include: ˙ One or more clusters involving a small number of human cases, e.g. a cluster of <25 cases lasting <2 weeks. ˙ Appearance of a small number of human cases in one or several geographically linked areas without a clear history of a non-human source of exposure. Phase 5. Larger cluster(s) but human-to-human spread still localized. It shows increased virus adaptation to human body, but lacking absolute effective infection strength. At this phase, there may still be possibility to carry out the last containment measure by means of international cooperation. Possible scenarios include: ˙ Transmission within cluster(s) continues to occur. But total patient number does not increase rapidly. For example, a cluster of 25~50 patients which only lasted for 2~4 weeks. - 24 - ˙Transmission continue to occur, but patients are localized within specific region (such as remote village, school, military camp, off-shore island, etc). ˙ In a community with known cluster of event. Infection source of a small number of patients cannot be identified. Phase 6. Pandemic: increased and sustained transmission in general population. At this phase all countries should strengthen surveillance and responsive strategy. 1.4.2 Taiwan Classification of Pandemic Phases WHO has set up the classification according to virus variation from a global point of view. Different suggestions are provided to affected countries and non-affected countries respectively starting from phase A2. Therefore, preparedness and responsive measures should be different for foreign or domestic epidemic situations. DOH announced Taiwan’s own Classification of Pandemic Phases on 29 December 2004: Classification Activation time Detection of avian influenza virus H5 or H7 domestically or Phase O confirmed human cases of avian flu aboard. 1. Lowly pathogenic avian flu occurred in poultry domestically 2. Highly pathogenic avian flu occurred in poultry domestically Phase A1 Phase A2 Confirmed human-to-human cases aboard Suspected domestic cases of fowl/animal-to-human transmission, imported infection or lab infection Phase B Confirmed domestic human-to-human cases Phase C Large-scale domestic human-to-human transmission - 25 - 1.4.3 Comparison of Classification of Pandemic Phases between Our Country and WHO Taiwan Classification Phase 0 Detection of avian influenza virus H5 or H7 domestically or confirmed human cases of avian flu aboard. WHO Classification Inter-pandemic Phase 1 Low risk of human cases Phase 2 High risk of human cases Pandemic alert Phase A1 Phase A2 Confirmed human-to-human cases aboard Suspected domestic cases of fowl/animal-to-human transmission, imported infection or lab infection Phase B Confirmed domestic human-to-human cases Phase C Large-scale domestic human-to-human transmission Phase 3 No or very limited human-to-human transmission Phase 4 Evidence of increased human-to-human transmission Phase 5 Evidence of significant human-to-human transmission Phase 3~ Phase 5 are all possible. Phase 5 Evidence of significant human-to-human transmission Pandemic Phase 6 - 26 - Efficient and sustained human-to-human transmission 2. Surveillance 2.1 Rationale The objective of surveillance is to continuously collect, analyze and announce information to control the epidemic. The Global Influenza Surveillance Network of WHO provides information on international epidemic situations. Domestically, CDC has established National Influenza Center (NIC) and multiple surveillance systems to understand the prevalence and variation of influenza virus at any time. When the domestic classification of pandemic alert is phase 0 and international classification is phase 3, information on epidemic situations overseas can be obtained through WHO network, IHR focal point and other established information exchange channels. Other than these, Bureau of Animal and Plant Health Inspection and Quarantine of the Council of Agriculture has established “SOP for the Surveillance, Alert and Reporting of Highly Pathogenic Avian Influenza (HPAI)”. Monitoring of avian flu was performed on suspected wild birds, farm poultry and excretion of migratory birds. Health authorities have also conducted surveillance on severe influenza cases and influenza cluster events. If abnormal condition is identified, investigation will be proceeded. Examination will be done when necessary to clarify the aetiology and to understand the trails of the new type of virus as early as possible. Criteria for “Human H5N1 influenza” case reporting, specimen collection and laboratory testing were set up according to information on H5N1 infection in humans overseas, in order to understand whether there are domestic cases of H5N1 infection in humans. Furthermore, lab surveillance system analyzes the variation of influenza virus to understand if the compositions of seasonal influenza vaccine can effectively prevent influenza occurrence. If domestic classification of pandemic is phase A1, international classification is either phase4 or phase 5, then health surveillance of incoming - 27 - passengers will be the major task of epidemic surveillance, other than obtaining information on the latest epidemic situations in affected countries through various channels. In the meantime, every domestic surveillance system should also increase alertness to monitor closely of any possible case in Taiwan. If domestic classification of pandemic becomes phase A2 and phase B (i.e. several H5N1 human cases or small clusters have occurred domestically), now the priority of surveillance is to identify all possible patients and to complete lab diagnosis promptly, so that epidemic control measures can be implemented immediately to block the virus from spreading, and to evaluate the outcomes of containment measures. During this phase, rapid test method is to be devised to shorten examination time. Should domestic classification upgrade to phase C and international classification to phase 6, in the beginning, surveillance system will still proceed with reporting and examination on a case-by-case basis. Characteristics of virus strain should be understood to facilitate the establishment of a gene database of the virus. Once it is found that most cases are human H5N1 infections, for which case by case examination can no longer help much with epidemic surveillance, but will instead increase infection risk for sampling staff, it may be possible that case by case examination and investigation will not be carried out in a later stage. At that time, existing surveillance system will be used to monitor the long-term trend of pandemic situation. Samples will be collected from a small number of patients for examination to understand variation of virus. Epidemiological research will be carried out at the same time to describe related characteristics of domestic influenza pandemic. In order to detect epidemic situation of avian influenza and pandemic influenza in time, so as to benefit from early alert, DOH has set up reporting procedures and investigation methods according to Article 4 and 26 of the Communicable Disease Control Act. Special municipality and county/city - 28 - governments should also implement measures for the surveillance, reporting, investigation, and management of epidemic situation in their respective jurisdiction according to Article 4 and 41 of the Communicable Disease Control Act. 2.2 Implementation Strategy 2.2.1 Usage of Multiple Surveillance Systems 2.2.1.1 Current Surveillance Systems Among multiple reporting channels set up by CDC, influenza related sections are described as follow: 1. Notifiable Diseases Surveillance System: The disease under surveillance is “Influenza with severe complications”. Reported cases will be examined by clinical expert and sample collected for testing. 2. Syndromic Surveillance System: “Acute respiratory syndrome” and “Acute neurological syndrome” reported by all hospitals above the regional level and by all local teaching hospitals. 3. Symptom Surveillance System: Patients that meet the case definition of “Person under investigation for Human H5N1 influenza” and cluster of influenza-like illness are to be reported through this channel. 4. Contracted Virus Lab Surveillance System: There are approximately more than 100 fixed sites for collection of samples. Two samples are selected each week and are sent to the 12 contracted viral infection labs to be tested for pathogen, in order to know the strain type of influenza virus. This provides a reference for making disease prevention and vaccination policy. 5. Surveillance System for Population Institutions: Management and health care providers of densely populated organizations should report within 24 hours of detecting any inhabitant or staff in the organization meeting any - 29 - criteria defined in “Standards for Immediate Reporting”. 6. Sentinel Surveillance System: There are in total around 700 sentinel physicians. They will report influenza-like patient numbers by phone, fax, email or mail, which enables understanding of the epidemiological trend of influenza-like cases country-wide and the possibility of an outbreak. 7. School-based Surveillance System: Given that schools are major transmission places of influenza, influenza-like cases will be reported each week. At present, there are more than 400 schools included in this system, with at least one public elementary school in each of the 25 counties/cities. Schools at all levels should report when ever a cluster occurs. In addition, DOH will compare influenza patients against a list of fowl and animal traders and farm workers provided by the Council of Agriculture. Once a trader in the list is identified as influenza-like illness, health authorities will be notified for special care. Fever patients found by border quarantine will also be reported and managed through Symptom Surveillance System. 2.2.1.2 Operation of Surveillance Systems When domestic pandemic is in phase O, and international pandemic is in phase 3, all surveillance systems maintain original operation in principle. Reported cases will be examined and be collected of samples. If abnormal clusters of patients occur, or epidemic situation worsens abnormally, further investigation should take place. Samples should be collected to clarify the pathogen. In this phase (phase O+phase 3), virus is not yet adaptive to human, therefore most infectious cases have contact history with fowls. Doctors and primary public health staffs should be reminded to ask patients in details about contact history with fowls and animals and travel history. If international pandemic enters phase 4 or phase 5, domestic pandemic will - 30 - be raised to phase A1. Border control will be the major measure. Incoming passengers from affected foreign areas must practice health self-management. Local health authorities must be aware of their health condition through system. Influenza patients with travel history to affected areas should be investigated and followed up with attention. Once a domestic case occurs, the domestic pandemic alert will enter phase A2 or higher. Reporting frequency of some surveillance systems will be increased in view of epidemic control needs, in order to control the expansion of epidemic situation timely, or to evaluate the effects of epidemic control interventions. DOH will revise reporting criteria immediately according to new information on human cases of H5N1 aboard as announced by WHO. The virus is not completely adaptive to human, hence future variation with different manifestation is still possible. Therefore reporting or sample collection criteria should be revised at any time according to information announced by WHO. In order to effectively apply surveillance information to the evaluation of control measures, integration of all epidemic information is necessary. Data in current systems are connected. An integrated information platform will be built up in the future. 2.2.2 Laboratory Diagnosis 2.2.2.1 Techniques of Laboratory Diagnosis Current techniques and methods used for laboratory diagnosis are: 1. Real time RT-PCR: If result is A(+) then continue with H1 & H3 subtype testing. If H1(+) or H3(+) then continue with sequence analysis. If H1(-) and H3(-) then continue with H5, H7, H9 subtype testing. 2. Virus culture: If cell culture of virus is positive, then continue with HI - 31 - method for serotype and gene sequence analysis of the virus strain. Influenza rapid test should still be confirmed by RT-PCR. Therefore it is not suggested unless in off-shore islands or cluster events. In current practice, patients who fulfill human H5N1 reporting criteria should be collected of pharyngeal-laryngeal swab, blood serum of both acute phase and recovery phase. According to US CDC Health Update (June 07, 2006), lower respiratory samples among all H5N1 samples have higher density of virus that is easier to be detected, including tracheal aspirates and bronchoalveolar lavage. These two samples can be collected in addition. 2.2.2.2 Execution of Laboratory Diagnosis At present, there are 12 contracted viral labs country-wide other than the NIC of CDC to execute the testing. The mechanism for collection of specimens and delivery to influenza testing labs has been set up and in running, and the labs will continue to maintain their testing capacity and biological safety. Should epidemic situation worsens in the future, the daily volume of samples handed can be increased in view of epidemic control needs. Coordination plan for deployment of back-up lab staff has also been devised in response to demand of large quantity of testing at that time. 2.2.3 Case Investigation The objective of case investigation is to learn about the infection mechanism, clinical manifestation and transmission strength of virus. According to information obtained from investigation, we can propose or revise self-protection methods timely, and to decide appropriate objects of prophylaxis and other containment measures. - 32 - 2.2.3.1 Execution of Case Investigation When in the stage of Phase 0 (domestic) and Phase 3 (international), local health authorities proceed with brief investigation after receiving a case report of human H5N1. If after testing, the case is found to be infected by Influenza A virus, but not H1 or H3 subtypes, a comprehensive investigation form should be completed within 24 hours. Investigation items in the form include: travel and exposure history 10 days before falling ill, investigation of contacts, clinical symptoms, medical history, etc. The “close contacts” identified will be included in the health self-management information system, and their condition will be managed and followed up everyday. “Close contacts” regarding “human H5N1 influenza” may change according to scientific knowledge. In principle, people who inhabit in the same house or have meals with a suspected patient of human H5N1 influenza during the period of communicability, office co-workers working within a radius of 3 meters, classmates, care-givers, riders on the same long-route (longer than 1 hour) public transport, etc., are recognized as “close contacts”, but the investigators can make flexible determinations according to actual investigation results. 2.2.3.2 Management of Close Contacts If the close contacts described in previous section had appropriate protection during contact with suspected patient, daily activities can still be maintained. If close contacts didn’t have appropriate protection during contact period, the possibility of being infected cannot be ruled out. Therefore arrangements should be made to decrease the opportunity of pathogen spread. Close contacts will be asked to perform health self-management for 7 days. During this period until being removed from health self-management, one should stay home and - 33 - avoid going out, maintain good hygiene habits, monitor one’s own health condition, and once a health problem develops, medical help should be sought according to instructions from local health authorities. If close contacts develop symptoms when being investigated, the possibility of human H5N1 infection should be considered. They should be arranged to seek medical help to clarify the cause of symptoms. - 34 - 3. Antivirals Strategy 3.1 Rationale There are 2 classes of antivirals specific for influenza: M2 inhibitors and neuraminidase inhibitors. M2 inhibitors launched earlier and are cheaper. But evidence shows higher incidence of resistance, and whether they’re safe to be used by pregnant women is unknown. A major concern is that H5N1 virus has been found to be resistant to M2 inhibitors. It is possible that H5N1 virus keeps the resistance and becomes global pandemic. Neuraminidase inhibitors, such as Oseltamivir and Zanamivir, are newly developed. Their safety profiles are relatively higher and resistance is relatively low. However, they are expensive and current supplies are very limited [9]. Antivirals are being used to treat and prevent seasonal influenza. In terms of responding to a influenza pandemic at the early phase, when effective vaccine is not yet available, the intervention of antivirals is extremely important. Most human H5N1 influenza cases were treated with oseltamivir. WHO has also stockpiled 3 millions doses of oseltamivir to contain the widespread of human-to-human transmission. However, the experience of oseltamivir treatment of H5N1 virus infection is not yet sufficient. There should be more research regarding its dosage and duration of administration. WHO suggests the following opportunities for using antivirals: (1) At present situation, antivirals are being used to treat H5N1 infected patients, and prophylaxis in patient’s close contacts such as health care workers and family members; (2) At the beginning of efficient human-to-human transmission, antiviral administration to the entire community where clusters are occurring may stop the virus from further improving its transmissibility or delay the spread; (3) If global influenza pandemic occurs, antivirals will be used as a medical intervention for reducing morbidity and mortality[9]. The stockpile and administration of anti-virals of DOH are planned according to above principles. - 35 - 3.2 Implementation Strategies 3.2.1 Stockpile Quantity of Antivirals Our country follows the principle of diversified stockpile. The medications chosen are neuraminidase inhibitors recommended by WHO. One is Tamiflu (Roche) with active ingredient oseltamivir, in both capsule form and API (active pharmaceutical ingredient). The other is Relenza (GSK) with active ingredient zanamivir, in spray dosage form. The total stockpile quantity is 2.37 million doses up to June 2006, which can cover at least 10.44% of population. The stockpile of these two medicines not only serves to avoid the occurrence of resistance, but can also be used in patients with special indication. Tamiflu API has the same active ingredient of Tamiflu capsule (oseltamivir phosphate). Several characteristics of the API, such as small volume (1 gram of powder is sufficient for 1 treatment dose), long shelf life (currently 5 years, can be extended to 11 years at the longest with proper storage), and rapid and large quantity prescription make it suitable to be used in treating large number of patients and in mass prophylaxis for rapid containment. In addition, Tamiflu API can be used in a wider range of age. All those who meet administration criteria of being older than 1 year of age can use this medicine. In order for Tamiflu API to be effective during influenza pandemic, DOH authorizes Taiwan Association of Clinical Pharmacy to hold “Anti-virals Prescription Training and Delivery Program”. Up to now, 105 prescription facilities have completed this training. 3.2.2 Time of Administration At the stage of phase 0 (domestic) and phase 3 (international), a patient who meets definition of human H5N1 influenza can be administered with antiviral immediately according to the patient’s physiological condition after being examined and sampled by a doctor and being reported to the health - 36 - authorities. Prophylaxis can only be provided to the workers involved in culling animals of HPAI. Upon entering phase B, C, Executive Yuan will establish a Central Epidemic Command Center (“Command Center” in short). Once the command center decides to use Tamiflu API, the logistics division of command center will inform the director of Taiwan Association of Clinical Pharmacy to activate prescription operation. The Logistics division will also inform Roche Pharmaceuticals to follow its instruction in delivering Tamiflu API in batches to GMP manufacturers contracted by DOH for packaging and labeling. GMP manufacturers will then deliver Tamiflu API to prescription facilities authorized by Taiwan Association of Clinical Pharmacy to proceed with liquid prescription. At last, the local governments will deliver the liquid drug. Drug prescription, delivery and use are controlled by the Material Information System (MIS, http://mis.cdc.gov.tw). 3.2.3 Administration Plan of Antivirals in the Future At the stage of phase A1/phase 4-5, when the virus is developing greater ability of human-to-human transmission abroad but no cases have occurred domestically, medicine recipients are still limited to patients who meet the definition for case reporting. Tamiflu capsules will be administered by medical organizations that perform testing and examination. At the stage of phase A2/phase 3-5, when there are suspected cases of fowl-to-human transmission, imported infection or lab-acquired infection domestically, medical organizations that perform testing and examination will provide Tamiflu capsules in a similar way to patients who meet the reporting criteria. Close contacts of suspected patients will be given Tamiflu capsules as prophylaxis. - 37 - At the stage of phase B/phase 5, when localized outbreaks have occurred in the country, Tamiflu capsules will continue to be supplied to patients who meet the reporting criteria. In addition, packaging and prescription of Tamiflu API will be performed according to the decision of the Command Center to facilitate extensive administration of the drug prophylaxis to block the virus spread. At the stage of phase C/phase 6, when the world has entered the pandemic phase with widespread domestic outbreaks, antivirals will be used to treat infected patients to prevent serious symptoms and to reduce fatality. All Tamiflu API will be packaged and be distributed by direct municipality and county/city governments to designated Infectoius Disease Prevention and Control Hospitals and to large-scale medical care facilities in their respective jurisdictions. The above-mentioned time of administration of Tamiflu is listed in Table 3.1. Relenza is for administration to the respiratory tract by local inhalation. It will be provided to health care workers of H5N1 influenza patients. 3.2.4 Procedures of the Use of Tamiflu API 3.2.4.1 Activation Mechanism for Use When entering phase B, the logistics division of central epidemic command center will propose to the commander about the activation mechanism of Tamiflu API use (proposal includes time of activation, quantity of prescription, demanded quantity of each county/city, and timeframe for completion). Once commander orders the activation of the mechanism, the logistics division will execute the following measures: 1. Inform Roche Pharmaceuticals by phone or email to deliver a specific quantity of Tamiflu API along with a medicine delivery checklist to GMP manufacturers designated by DOH within a specific period of time. - 38 - 2. Inform the GMP manufacturers by phone or email to await order to proceed with packaging and labeling. Ask the manufacturers to check the quantity of Tamiflu API. If no discrepancy is noted, fax the medicine delivery checklist to the logistics division and confirm by phone. 3. Inform Taiwan Association of Clinical Pharmacy by phone or email about this prescription order and contents. Ask them to report back when standby is ready by phone or fax and to report regularly on the progress of prescription in each area. 4. Inform each local government in writing about the activation of the Tamiflu API mechanism. Also inform them about the estimated time to get Tamiflu solution at prescription facilities in respective county/city. 3.2.4.2 Packaging and Labeling 1. The warehouse of Roche Pharmaceuticals will complete delivery of API to contracted GMP manufacturers within a specific period after receiving delivery notification from the logistics division. 2. GMP manufacturers will complete all API packaging and labeling within 72 hours, and deliver the medicine to contracted prescription hospitals. 3.2.4.3 Medicine Prescription 1. Prescription facilities receive small packages of Tamiflu API. After confirming no discrepancy is noted, log into MIS and enter Tamiflu API into the system. 2. Prescription facilities start prescription procedure according to indications of Taiwan Association of Clinical Pharmacy. After the completion of prescription, enter the quantity of Tamiflu solution into MIS to complete the procedure. - 39 - 3. Taiwan Association of Clinical Pharmacy reports back regularly to Logistics about the progress of prescription of each area by phone or fax. 4. Logistics and local responsive center obtain information on prescription status in MIS. 5. Prescription facilities are responsible for proper storage of medicine stored in their facilities. 3.2.4.4 Delivery 1. Tamiflu API is used according to command center’s indication or by local command center’s decision in its governance based on epidemic situation. 2. The local governments should arrange vehicles and staffs to prescription facilities to receive medicine after presenting a medicine requisition list with authorization of Bureau of Health. 3. After receiving the medicine, store it under 25℃ and deliver it promptly to places that demands the medicine (large-scale care facilities, infection prevention hospitals, communities or other). Ask them to check and accept the medicine and to provide an administrating list. 4. Bureau of Health will log data into the MIS according to the administrating list. 5. If cross county/city administration and delivery of medicine are involved, local branches of CDC will coordinate it. 3.2.5 Information Management MIS is used as the operation interface for managing information flow on anti-virals, and this applies to all three medicines stockpiled in our country (Tamiflu capsule, Tamiflu API, Relenza). Recipient units of the drugs make - 40 - entries into MIS, and handouts are also registered into the MIS by the units when medicine is sent out. The drugs are handed down units, which must subsequently report back the basic information of drug recipients. 3.2.6 Activation of Domestic Manufacture of Tamiflu The office of Intellectual Property of Ministry of Economic Affairs has granted special permission to DOH to overlook the patent of Tamiflu under certain conditions since November 2005. The type of Tamiflu manufactured in our country is in API form. The administration procedure is the same as “3.2.4 Procedures of the Use of Tamiflu API”. The domestic manufacture mechanism is activated by the decision of commander of the Command Center according to epidemic situation and the storage quantity of anti-virals during influenza pandemic period. - 41 - Table 3.1 Time of Administration of Antivirals Source of Medicine Phase Recipient Tamiflu capsule Phase A1/ phase 4~5 1.Human-to-human cases aboard 2.No domestic case Phase A2/ phase 3~5* Suspected domestic cases of fowl/animal-to-human transmission, imported infection, lab-acquired infection Phase B/phase 5 Localized domestic outbreaks Those who meet definition of human H5N1 influenza Sentinels for H5N1 infection 1.Those who meet definition of human H5N1 influenza 2.Close contacts of possible cases 1. Sentinels for H5N1 infection 2.Local Branches of CDC 1. Those who meet definition of human H5N1 influenza Sentinels for H5N1 2.Large-scale infection administration of preventive medication aiming at regional containment of virus Phase C/phase 6 1. Large-scale 1.Global pandemic administration of 2.Widespread domestic preventive medication outbreaks aiming at containment of virus 2.Patients treated at infection control hospitals 3.Patients treated at large care facilities - 42 - Tamiflu API Central Coordination 1.Central Coordination 2.Central or local government coordination 3.Central or local government coordination 4. Vaccine Strategy 4.1 Rationale Influenza vaccine is the most important means to control influenza epidemic. Even though seasonal influenza vaccination cannot prevent future influenza pandemic, the routine promotion of the annual influenza vaccination program contributes to capacity-buidling in the manufacture, delivery and administration of pandemic vaccine. Our country’s current vaccination program aims at elders above 65 years of age, infants between 6 months and 2 years of age, health care personnel, workers in industries related to poultry and animal husbandry and animal influenza control workers. At present, all vaccines are purchased from international manufacturers through agent. We are temporarily unable to manufacture influenza vaccines. Traditional manufacture of influenza vaccine uses egg-based technique, which requires large quantity of eggs. Cell-based technique is more advanced, with the advantage of more capability of manufacturing large quantity of vaccine in response to epidemics. Cells to be used can be stored frozen in advance, lest the decrease in egg quantity caused by avian flu will impact the manufacture of vaccines. People with allergies to eggs can also be vaccinated. The US Government announced an investment of $1 billion in May 2006 to develop research of cell-based technique and vaccine manufacture.[10] Based on current vaccine manufacturing technique, once influenza pandemic occurs, mass production of pandemic flu vaccine will become possible 6 months after acquisition of virus strain. WHO have invited related experts and vaccine manufacturers to discuss relevant issues concerning vaccine research/ development and supply, hoping to shorten the period of vaccine manufacture and supply. Even so, it is not easy for countries with no vaccine manufacture capacity to obtain sufficient pandemic vaccine at the beginning. Influenza vaccine - 43 - manufacturers are located in 9 countries: Australia, Japan, Canada, USA, France, Germany, Italy, Netherlands and United Kingdom. In 2003, global vaccine supply was approximately 292 million doses. 71% among them was provided to Australia, Japan, Canada, USA and Western Europe countries. That is to say, with only 12% of the world’s population, these vaccine manufacturing countries used almost 2/3 of influenza vaccine. According to WHO estimation, 70% of global influenza vaccine is manufactured by 5 major European manufacturers. However, the most probable origin of influenza pandemic virus –Asia, is located at the far end of vaccine supply. There are enormous differences between supply and demand whether in total manufacturing capacity or region distribution. Once pandemic occurs, countries without manufacturing capacity will be unable to acquire sufficient vaccine. Our country does not possess the manufacturing technique and capability of influenza vaccine now. Facing an unknown future, if influenza pandemic occurs, global demand for vaccine will increase substantially hence causing imbalance of supply and demand. At that time, importation of sufficient vaccine cannot be guaranteed and will have huge influence over every aspect of our country. In view of that, it is essential and important for Taiwan to acquire manufacturing techniques capacity for influenza vaccine. Taiwan should even develop influenza vaccine related industries to improve national epidemic control and response capabilities. To effectively implement the “Influenza Vaccine Self-Manufacture Plan” approved but Executive Yuan in November 2004, and to build up domestic vaccine manufacture capability, CDC has launched a “BOO Project for Domestic Manufacturing of Influenza Vaccine for CDC” according to the “Law for Promotion of Private Participation in Public Infrastructure Project” (“Law for Promotion of Participation” in short). - 44 - 4.2 Implementation Strategies 4.2.1 Short-Term: Stockpile of “Pandemic-like” Vaccine To provide sufficient vaccine during influenza pandemic period is one of the major objectives of CDC. To be farsighted, the most fundamental solution is to establish the capability of manufacturing influenza vaccine. However, the timing of influenza pandemic onset cannot be estimated precisely. If it comes within a short time, stockpiling foreign-made H5N1 vaccine which is under clinical trial for emergency will be the way to respond. Therefore, with the CDC striving for funds, Executive Yuan agreed in 2006 on a budget of USD $1.9 million to purchase and stockpile human influenza A/H5N1 vaccine. Arrangements concerning purchase and public bidding are underway to acquire doses to cover at least 95,000 people. The stockpile will be provided to first-line health care workers and epidemic control staffs with priority when influenza pandemic occurs. 4.2.2 Medium-Term: Self-manufacturing of Emergency Vaccine In order to effectively deal with influenza pandemic, CDC has solicited and elected publicly for research and development plans of influenza vaccine in our country according to Government Purchase Law. And it will be executed by public and private colleges and universities, public academic research organizations, corporate academic research organizations, medical facilities with ranking higher than regional hospital, and medical health related academic organizations. Execution period is 2006-2008, with total funds of USD $18.8 million. Funds of 2006 Influenza vaccine research and development plan are USD $4 million. It can be divided to 3 major frameworks and 20 plans. The contents are: 1. Selection of vaccine strain and vaccine policy research - 45 - (1) To manufacture standardized antiserum and antigen for influenza virus (2) Research on Taiwan influenza virus molecular evolution and the influence of each gene on virus antigen manifestation (3) Analysis of influenza virus HA and NA:Integration of Taiwan influenza virus detection systems, in order to set up standard procedures and methods for collection and analysis of influenza virus information (4) Establish bioinformatics system of influenza virus (5) Utilization of integrated infectious disease surveillance software to assist the monitoring of influenza and to provide public health reference (6) Use mathematical models to research Taiwan’s influenza vaccine policy and other disease control policies. (7) Establish the blood serum database of community public and monitor antibody immunoreactions before and after influenza vaccination in Taiwan children, adults and elders (8) Influenza vaccine research and development plan— Epidemiology sub-plan: Establish our country’s surveillance system on antibody to influenza virus (9) Establish our country’s surveillance and analysis system on antibody to influenza virus— Evaluation of antibody reaction after vaccination and analysis of immunity condition of people with poor antibody reaction after vaccination (10) Investigation of serum antibody to human influenza A virus and avian flu virus of poultry/animal husbandry workers in Taiwan and Kinmen area 2. Establish Basic Vaccine Technique (1) Influenza virus group study—Interaction of congenital immunity receptor and virus, technical platform for immunity analysis, influence of virus - 46 - gene variation to immunity reaction of host, manufacturing of primitive virus vaccine (2) Identification of genomes of new influenza virus strain and manufacture of new vaccine strain by reverse genetics (3) Influenza virus group study—virology, immunopathology, technical platform for immunity analysis, technical application of reverse gene system (4) Emergency plan of influenza vaccine manufacturing (5) Manufacture of new influenza vaccine using serum-free micro carrier cell culture (6) Research and development of new influenza subunit vaccine and new high-molecular vaccine slow release form (7) Improve mammal cells and select virus replication accelerator to increase the capacity of influenza virus (vaccine strain) (8) Research and develop bi-valence vaccine combining human influenza and avian flu using new type carbohydrate and lipid assisting agent. 3. Promote the development of this industry and the execution clinical trials (1)Establish our country’s management mechanism of clinical studies of new influenza vaccine (2)Development plan for technical and managerial talents The establishment of our country’s emergency manufacturing line of new influenza vaccine is planned and executed by National Health Institute. This plan is expected to start in the 3rd quarter of 2006, and will entail continuous manufacturing and stockpiling of H5N1 influenza vaccine. 5000 doses of H5N1 vaccine to be used in clinical trials will be available in the 4 th quarter. IND application of clinical trials will begin at the 2nd quarter of 2007. Clinical study - 47 - will start in the 3rd quarter of the same year. It is expected that emergency manufacturing capacity can reach 100,000 doses per year in the 3 rd quarter of 2008. 4.2.3 Long-Term: Plan for Domestic Vaccine Manufacture DOH has enthusiastically raised funds to conduct domestic manufacturing of influenza vaccines. The tender process for seeking influenza qualified vaccine manufacturers has been completed. It is estimated that, at the end of 2009, our country will have its first flu vaccine plant with an annual manufacturing capacity of 16 million doses. In the future, this factory must have an annual production of at lease 16 million doses of general tri-valence influenza vaccine. When influenza pandemic occurs, it should be able to provide vaccines that cover at least one fourth of Taiwan’s population within 3 months. In view of the government’s obligation and responsibility to establish epidemic prevention system, civilian participation is introduced to the execution aspect of domestic manufacturing of influenza vaccine to improve the efficiency and quality of public construction. Here the Law for Promotion of Participation is used. A civilian organization invests and constructs the new facilities. The organization has ownership, and can manage the business by itself or through a third-party (Build-Own-Operate, BOO in short). In order to encourage active participation of private manufacturers, long-term purchase agreement is planned as a strategy to encourage investment other than methods defined in Law for Promotion of Participation. As for the timeframe, the contract is estimated to be signed in the 2 nd half of 2006. Factory construction will be completed 2 years within signing the contract. Production will start within 3 years of signing the contract. - 48 - 4.2.4 Establishment of Capability in Virus Strains Selection Because of the frequent antigen draft of influenza virus, WHO give suggestions to north and south hemispheres respectively every year on influenza virus strains of vaccine to be administered. The virus strains chosen are based on virus strain information and epidemiologic trends collected by WHO Global Influenza Surveillance Network. The Network includes 116 national influenza centers in 87 countries, and 4 WHO Collaborating Centers (US, United Kingdom, Australia, and Japan). These participating centers process samples from influenza-like patients for virus testing, and send representative isolated strains to a WHO Collaborating Center to continue with gene and antigen analysis. In order to establish our capability of vaccine manufacture, it is also essential to establish the ability to identify and select virus strain, other than research and development of techniques and factory construction plan. Besides existing resources, cooperation with internal and international scholars is also necessary. 4.2.5 Administration of Pandemic Vaccine Some research institutes and vaccine manufacturers are working on technique development of related vaccine. Some are in clinical trial stage. We are still waiting for a breakthrough in aspects of increasing immunity and development of evaluation tools. How to speed up procedures like registration for examination when a pandemic takes hold is a major topic for both foreign and domestic organizations. As for the recipients of pandemic vaccination, medical staffs, personnel who maintain essential social functions, high-risk population who tends to develop serious symptoms or death, will be vaccinated with priority considering available vaccine quantity at that time. - 49 - 5. Transmission Interruption Measures 5.1 Rationale Traditionally, people all think vaccination is most cost-effective way to infectious disease control. However, as far as influenza pandemic is concerned, the influenza virus strain that cause pandemic is not available. It is not possible to manufacture effective and large quantity of vaccine timely to prevent occurrence of pandemic. Though WHO recommends antiviral stockpile for pandemic preparedness, the appropriate dose and administrative duration for pandemic flu is unclear. The occurrence time and scope of pandemic is unpredictable. As a consequence, the stockpile of medication is indeed troublesome. In addition to ”non-pharmaceutical public health interventions” described in WHO Global Influenza Preparedness Plan, [8] in Emerging Infectious Disease Journal, those interventions are categorized into 4 types: (1) Measures that limit the international transmission of virus, such as screening of fever at border and travel restriction; (2) Measures that reduce virus transmission, such as isolated treatment of patient, health self-management of contact, quarantine, cancellation of rallies and class suspension, etc; (3) Decrease personal risks, such as frequent practice of hand-washing; (4) Communication of risks to the public [11] . In our country, these prevention measures are generally called “ Transmission Interruption Measures”. In the “Implementation Plan for Pandemic Influenza” announced by the US Department of Homeland Security in May 2006, some social distance measures were mentioned, for example, keeping distance of at least 1 yard (3 inches) with others, using teleconference tools in meetings at work, closing elementary schools, canceling unimportant rallies, or restriction of traveling, etc. These measures are more cost-benefit. Other measures include snow day restrictions, namely that government force the public of staying home to limit social contacts - 50 - to reduce communicable diseases transmission. These measures cost more, therefore can only be implemented for a limited duration. But they there should be at least two incubation periods in order to reach maximum benefit. US CDC announced in October 2006 that it will take months to develop effective vaccine. Therefore “non-pharmaceutical interventions” could be the first line measures. Except for hand-washing and cough etiquette, it is of high priority to select appropriate prevention measures in community. These measures include “social distance” (closing schools/work places or canceling rallies) and “ isolation & quarantine”. Therefore once the pandemic occurs, low-cost and continuous social distance measures should be implemented immediately. High-cost and short-term measures can be hold back until there’s a need of containment.[12] The Spanish influenza pandemic in 1918~1919 almost killed 50,000,000 lives globally, with around 675,000 Americans. It was estimated that among American metropolis, the lowest mortality rate was 0.3% in St. Louis. The highest was 0.8% in Pittsburgh, and next was 0.76% in San Francisco. Why a lower mortality rate was in St. Louis? Public health scholars believed that it should be attributed to Dr. Starkloff, the health official of St. Louis at that time. He was alert that Spanish flu might invade along with troops. In early October he asked the mayor to implement social distance measures such as closing schools, theaters and churches, prohibition of rallies, balls at hotels and restaurants, restriction of public visit the sick in the hospital, and restriction of children going to playgrounds or libraries. After Dr. Starkloff died in 1942, the City Hospital of St. Louis was named after him to remember his contributions. When recalling past events, we should also learn from them to respond to pandemic. The scientific evidence of non-pharmaceutical interventions effectiveness in influenza prevention is quite limited. In fact, the guidelines are mainly obtained from observation of history and present day instead of control studies. - 51 - Recently mathematical simulation is added. Much information is from estimation and calculation. Therefore, during pandemic, epidemiology, virology and field investigation should be applied immediately to proceed with cost-benefit analysis. With estimation of mathematical simulation, a more objective and precise decision-making basis can be provided. There are 4 principles of using these measures: (1) Broaden the scope of crisis management; (2) Limitation of social interaction; (3) Using the fewest and necessary restriction measures; (4) Making community public into partners. There are 3 elements of infectious disease development: pathogen, susceptible host and transmission pathway. In a word, to prevent the spread of pandemic, the susceptible host should be prevented of contact with pathogen. Therefore, we can begin with eliminating or reducing infectious sources and stopping or slowing down virus transmission among people . Strategies of infection control and strategies of contact restriction are generally called “Transmission Interruption Measures “ in this text. According to Communicable Disease Control Act, central and local governments have their own responsibilities and authorities to implement prevention measures. All departments of government have started preparation actively in response to the pandemic threat. Transmission interruption measures constitute 1 of the 4 major strategies of pandemic prevention in our country. All levels of government must make all the people to cooperate in order to eliminate the occurrence, transmission and spread of infectious diseases. 5.2 Implementation Strategies During pandemic alert period, control measures such as surveillance, case investigation, patient isolation, contact tracing and prophylaxis with antivirals are intended to delay the occurrence of pandemic,. But during pandemic period, depending solely on the above measures will not necessarily prevent the - 52 - epidemic from spreading. Community-based concepts should be incorporated in the thinking of prevention measure application, for example closing schools, canceling public activities, constraints similar to snow day restrictions, quarantine of close contacts or even enlarged community quarantine (such as closing streets). The decision making and time consideration of broadening social distance measures should be evaluated according to epidemic situation of each individual community. No single measure can be applied to all epidemic situations. For this reason, the following measures can be implemented individually or in combination. All the infection control strategies and contact restriction strategies described in this chapter may be applied during a pandemic. When the time comes, national contact restriction strategies will be decided and ordered by Central Epidemic Command Center according to virus characteristics, prevention needs and feasibility at that time. Local governments, medical facilities and community organizations can fully understand the rationale and objectives of each measure through the content of this plan. The way of implementation can be planned in advance to make use of them with flexibility. 5.2.1 Infection Control Strategies Infection control is to employ physical measures to protect individuals from entering into or contact with pathogen-polluted environment. It is divided into 4 types according to disease transmission pathways: (1)standard precautions; (2)contact precautions; (3)droplet precautions; (4)airborne precautions. Details can be found in the newest version of infection control guidelines drawn by WHO, on subjects such as environmental cleaning and disinfection process, hand hygiene, cough etiquette, personal protective equipment (PPE) and operation procedure control, etc.[13] - 53 - Coughing, sneezing and speaking create droplets. Pathogens in mucous membrane of respiratory tract, such as H5N1 influenza virus, will probably adhere to the surface of droplets≧5 ㎛ in diameter and suspend in the air. Droplets will fall gradually to the ground or surfaces of environment facilities because of gravity over about a distance of 1 meter (3 inches). If one’s hand gets in contact with contaminated surfaces and then touches the mouth, nose, or eye without disinfection procedure, then virus will invade the mucous membrane and infects that person. If moisture of the droplet vaporizes which makes its diameter<5 ㎛, then the droplet core will float even farther under airflow influence. Virus infection through this method is called airborne transmission pathway, the extent of influence of which will be even greater. There is sufficient evidence in several studies that influenza virus is usually transmitted by tiny particles of respiratory tract rather than by larger ones as believed in the past. Different from large particles or droplets that may be trapped in upper respiratory tract, tiny particles are likely to invade the lungs. Much evidence shows that current H5N1 influenza virus mainly invades lower respiratory tracts. Human influenza virus can remain in smooth surfaces for about 24~48 hours. In the surfaces of clothes, paper or toilet paper (in the environment of 35~49% humidity and 28℃), it can remain for about 8~12 hours. For this reason, cleaning and disinfection are effective ways to reduce pathogen during pandemic. Cleaning should come before disinfection. In the infection control guideline of WHO, the following ingredients are suggested to be used: phenols, quadrivalence ethanol(70%). ammonium, Refer hydrogen to peroxide solution, following chloride, web and page: www.who.int/csr/disease/avian_influenza/guidelines/EPR_AM_final1.pdf In addition, for details of key points regarding hospitals’ work on infection control in response to avian flu and pandemic flu, refer to following web page: http://www.cdc.gov.tw/internet-cdc/疫情報導/files/第 22 卷/中文版 22-07.pdf - 54 - 5.2.2 Contact Restriction Strategies 5.2.2.1 Isolation Isolation is to separate and limit the movements of suspected, possible or confirmed cases of infectious disease within a specific facility with medical service provided, in order to reduce the possibility of pathogen spread. Regarding the duration of isolation, basically, it is determined by remission of clinical symptoms and virus characteristics, namely the duration in which a patient continues to release the virus and whether immunity is developed in patient. The planning of isolation during pandemic can be classified to: 1. Air isolation wards The planning of this kind of wards can facilitate influenza pandemic control. But practically the number of wards is limited after all, and cannot satisfy the needs of a large scale epidemic. All levels of government should establish and plan substitutes for isolation wards in community in advance. 2. Home isolation When hospital capacity is overloaded, home isolation is one of the options. Patient should be separated from family during home isolation treatment period. It has 4 advantages: With less pressure, more comfortable, more opportunities of family-provided care, and psychological benefit. There are also 2 disadvantages: increased risk of family infection and decreased accessibility of professional medical services. Therefore, if patient cannot obtain proper and basic medical services, home isolation should not be an option. Once home isolation is executed, necessary PPE and sufficient information should be provided to family and care-givers. Related discards produced by patients have to be handled properly. 3. Facility isolation When air isolation wards are greatly insufficient, or home isolation - 55 - could not be fully supported by family, requisition of a designated hospital could be the best option for facility isolation. When hospital requisition is not possible, other facilities can be taken into consideration, for example hotels, schools, gymnasiums, religious constructions, nursing homes, convention centers, mobile tents, ambulant trailers, sailing ships, government organizations, etc. Every operation procedure inside the facility should take into consideration the possibility of direct or indirect infection of staffs other than patients. If yes, the infection factor should be prevented or eliminated in advance. 5.2.2.2 Quarantine Those who are suspected of infection exposure but not yet falling ill will be asked to wear masks correctly to be differentiated and to limit their movements. They should keep a distance of at least 1 meter with others. The objective of quarantine is to monitor their health condition and to know well about possible patients as soon as possible. The quarantine duration is uniformly 7 days for close contacts. Quarantine cannot stop infectious disease transmission immediately, but it’s one of the many methods to decrease new patients. Both isolation and quarantine will restrict personal freedom. Most people can accept that when symptoms occur one should be isolated. However, quarantine performed on those under exposure but with no symptoms is difficult to implement, even with the consideration of public interest over personal interest. Even so, local governments should well communicate with people about what are the risks and what are government’s strategies. In addition, daily activities and food during quarantine should be pre-planned. According to characteristics and scale of epidemic situation, the following types of quarantine can be adopted: 1. Home quarantine - 56 - The subject unit of home quarantine could be individual or family. But only when most people in a building are at risks of exposure can we consider including all people in the entire building into subjects of quarantine. People who fall ill during quarantine period should be escorted to isolated treatment immediately. During the quarantine period, individuals under quarantine should wear masks correctly. Their daily activities, ranging from sleeping and eating to drinking and bathing, should be separated from others. Once a quarantined individual falls ill, in addition to being sent to treatment immediately, the patient’s family will become subjects of quarantine and their activities restricted. If home quarantine is regarded as necessary and appropriate, local government should ensure that special requirements of susceptible groups are met, including elders, infants, those with movement incapability, and those with chronic diseases. 2. Facility quarantine For those who will not or cannot sustain home quarantine, for example people without relatives, tourists or those requiring special care, local governments should set up quarantine facilities to take in these kinds of quarantine subjects and limit their movements. 3. Work quarantine Work quarantine may be implemented for health care workers. These personnel are most possible to be exposed to pathogen, and they play important roles in epidemic control. It will be troublesome to implement home or facility quarantine for them. On the other hand, work quarantine will allow staffs to continue to work, but PPE should be used. When not working, home or facility quarantine can be implemented, meaning that they cannot - 57 - have contact with family or other colleagues, and have to perform strict health self-management. Those who develop symptoms will be reported and be sent for treatment. 4. Community quarantine Community quarantine can be implemented in an area if, within this area, there are a relatively high number of confirmed cases or extensive exposure risks. All people in this area are to be restricted of their movements, such as closing of streets and hospitals during SARS period. 5.2.2.3 Community restriction Community restriction is the way to reduce public interaction in community through limitation of public activities and closing of buildings, etc. Community restriction may be implemented together with isolation and quarantine, but they are different in ways of execution after all. Community restriction measures not target individual cases or groups, neither will it target patients, contacts or potential contacts directly. Basically, the subject of implementation is one whole community. Social interaction within the community will be restricted to reduce possibility of disease transmission. Due to its great impact, it should be considered of how to execute to reach maximum effects, and how to maintain functioning of other important infrastructures as well. For example, if there are other transportation systems to substitute when mass transit system is closed. Below are 5 classes of restriction measures to be referred to when drafting implementation practice: 1. Encouraging community-based infection control actions For example: environment cleaning, practice of frequent hand-washing, cough etiquette, bowing instead of hand-shaking, and discouraging kiss - 58 - salutation, etc. 2. Travel restrictions Travel types include air, sea, railway and land route, etc. The extent of restriction ranges from issuing travel warning to stopping people from getting near high-risk regions to canceling trips. 3. Canceling public gatherings For example, sports games, performances, concerts, political rallies, festive activities, religious activities, weddings and funerals, etc. The principle is cancellation; minimization of scale and postponement as exceptions. 4. Closing public facilities For example, schools, government offices, transportation stations, libraries, and public swimming pools, etc. When thinking about closing civilian properties such as mega selling stores, concert halls, skating rinks, theaters and hotels, etc, there must be strong legal basis, such as an emergency order. Closing elementary schools will help reduce or prevent influenza transmission among young school children. But the need for someone to accompany the parents at home should also be considered when implementing, because we do not want to see children being let loose and gathering outside schools. 5. Reinforcement of screening capacity 5.2.2.4 Sheltering Sheltering is a measure to restrict many people’s social activity in order to protect their health. It is different from isolation and quarantine. It does not target those who fall ill or contacts. It targets those who have never been - 59 - exposed to pathogen; and it is not compulsory. It can be considered in below situations: (1) when the scale of community spread is as large that it’s impossible to proceed with contacts investigation, and with social activities continuing, the risk of infection will not be slowed down; (2) when isolation, quarantine and community restriction measures still cannot properly prevent pandemic flu from spreading; (3) when influenza virus is very contagious and is greatly pathogenic, all active measures should be implemented, even though some can only serve to limit the transmission of disease; (4) when the disease is infectious even before symptoms appear. In foreign countries, during the days of snowstorm attack, and in our country when typhoon attacks, government will announce “no work or no school” messages. The objective is to restrict people going out to ensure safety by asking people to stay home. These actions are not compulsory and the compliance is associated with the enhancement of people’s “self-sheltering” behaviors. They are usually called ”snow day restriction” or ”sheltering” or ”self-shielding” in literature. This “self-shielding” concept was first developed to be applied to management in response to biological terror events. Nowadays, this concept can also be used during pandemic period. After each county/city government makes such an announcement according to law, people should make voluntary decisions to stay home and decrease going out for public gatherings. The implementation period can be set initially as 2 weeks, after that, strategies can be refined according to epidemiological information at that time. Nonetheless, during pandemic, local governments should be cautious when making such decisions as to maintain basic social infrastructures, such as communication and transportation, water and electricity, etc. - 60 - 6. Preparedness of Personal Protective Equipment 6.1 Rationale In laboratories, out-patient consulting rooms, emergency rooms and isolation wards, infection control installations and measures should be considered in advance, and personal protective equipments (PPE) can be used to supplement insufficiency of those fixtures and measures. PPE is also critical to ensuring the safety of first-line workers who are involved in case investigation, health education and culling work in avian flu affected farms. Therefore, advance planning and implementation of PPE preparedness are urgently needed in the face of pandemic threat. Based on SARS control experience, the most important PPE is mask. According to material, common masks sold in the market can be roughly divided to: gauze masks, cotton masks, activate carbon masks and medical masks. The filtration rate of the first 3 types of masks is less than 20% for particles with diameters between 0.1 ㎛~1.0 ㎛. Medical masks can be divided into 3 types according to CNS14774 and T5017 standards: “procedure mask” (flat mask), “surgical mask” and “surgical D-2 dust-proof mask” (level of N95 or above mask). Their filtration rates are 70%, 80%, and above 95% respectively. Whether protection can be achieved depends on wearing a mask correctly and performing a fitness test whenever possible. P100 semi-face respirator and powered air purifying respirator (PAPR) are used for aerosol-generating procedures when having close contact with patients (within 1 meter of distance) with high frequency, or when performing endotrachel intubations treatment. DOH and all local governments are both asked to stockpile PPE respectively according to Articles 4.1.1.1 and 4.1.2.2 of Communicable Disease Control Act. Medical facilities also have to stockpile sufficient PPE by themselves according to Article 20 of the same law. - 61 - 6.2 Implementation Strategies 6.2.1 Estimation of PPE Demand The main objective of estimation for the overall demand is to provide reference for drafting inter-entity supply contracts of related items and for estimation of related budgets. Estimation is done by using FluAid2.0 and FluSurge1.0 software developed by USCDC to predict the curve of infections, out-patient visits, hospitalizations, mild cases, serious cases and death numbers under the scenario of influenza pandemic with an attack rate of 25% to 35%, from which demands for various epidemic control materials can in turn be derived. Among them, the demands of the Medical Network for Prevention and Control of Infectious Disease in early phases are calculated according to medium attack rate (25%). Demands of large care facilities in later phases with the most serious epidemic situation are calculated according to high attack rate (35%). Parameters of PPE rundown are determined by referring to related WHO guidelines and the advice of infectious disease experts in our country. 6.2.2 Determination of Safety Stockpile According to the above estimation and the PPE market conditions (both manufactured and imported), once pandemic shifts to phase C, there will be significant difference between supply and demand of all major types of PPE in Taiwan. Chart 6.1 illustrates the integration of the difference, which is the safety storage volume. In addition, based on the quantity of each PPE item consumed by the central government, local governments and medical facilities in the recent two and half years after SARS epidemic, safety stockpile volumes at these three levels have been established. Central government’s stockpile is for epidemic control and emergency dispatch. Local governments’ stockpiles provide for local public health and epidemic control needs. Therefore, 25% of the total safety stockpile comes from the central government, and local governments are - 62 - responsible for another 25%, with the remaining 50% of total safety stockpile shared by medical facilities. Table 6.1 shows the estimations of safety storage and distribution list. Chart 6.1 Safe Stockpile of PPE Table 6.1 PPE Stockpile and Allocation Levels Items N95 equivalent or higher-level masks (pcs) Surgical mask (pcs) Flat mask (pcs) Protection clothes (suit) Central Local government governments Medical facilities Total 500,000 500,000 1,000,000 2,000,000 1,750,000 1,750,000 3,500,000 7,000,000 75,000,000 0 200,000 200,000 0 75,000,000 400,000 800,000 6.2.3 Information Management and Monitoring The goal of PPE management is not only to set up safety stockpiles, but also to grasp real-time information about the dynamic changes in PPE stockpiles to provide accurate parameters for the reference of decision makers. Stockpile - 63 - monitoring is used to manage abnormalities and to prevent gaps between registered information and actual storage. 6.2.3.1 Real-time Information 1. In phase 0, each storage unit logs into Material Information System (MIS) at least once every 2 weeks to upload updated information on use, replenish and storage. 2. In phase A, each storage unit logs into MIS at least once a week to upload updated information on use, replenish and storage. 3. In phase B and phase C, each storage unit logs into MIS everyday to upload updated information on use, replenish and storage. 6.2.3.2 Stockpile Inspection and monitoring for Abnormality Management 1. Primary inspection: For those who did not log in within time limit or when a shortage of storage is detected, the system will issue an automatic notification email to the reserve manager on the first day. System maintenance personnel will telephone to ask for improvement on the second day. 2. Secondary inspection: For those who did not log in within time limit or when a shortage of storage is detected, the local health bureau will intervene to inspect from the third day to the ninth day. 3. Tertiary inspection: For those who have exceeded the log-in time limit or have had a shortage of storage for over 10 days, the regional branch of CDC will supervise the local health bureau in reinforcing inspection. Direct intervention of inspection will be implemented when necessary. - 64 - 6.2.4 Reallocation and Delivery 6.2.4.1 Reallocation 1. Active dispatch: When central and local governments become aware of PPE shortage in related units through MIS, active deployment will be arranged after evaluating the reserve condition and confirming with the units with shortage. 2. Application for dispatch: Those who have shortage in PPE reserve can apply for dispatch from central and local governments. The application will be either approved or rejected after evaluating the epidemic situation and reserve condition. Hospitals can also apply for support materials from nearby hospitals in similar way. 3. The payment for material deployment or the arrangement for free dispatch is agreed between the supporting and the supported institutions unless otherwise defined by law. 6.2.4.2 Delivery 1. Ordinary delivery: Ordinary delivery from central government to local governments and hospitals is limited to 2 days after approval in main island and 3 days for off-shore island. Nearby delivery from local governments to medical facilities is in principle limited to 24 hours after approval. Ordinary delivery timeline between hospitals is as agreed by both parties. 2. Emergency delivery: Emergency delivery from central government to local governments and hospitals is limited to 24 hours after approval in main island and 3 days for off-shore island. Nearby delivery from local governments to medical facilities is in principle limited to 4 hours after approval. Emergency delivery timeline between hospitals is as agreed by both parties. - 65 - 3. The delivery can be carried out with civilian support. Requisition and compensation can be processed when necessary according to “Epidemic Control Materials Requisition Operation Procedures and Compensation Method”announced on July 14, 2004. 6.2.5 Dealing with PPE Shortage 6.2.5.1 Reduce Expenditure 1. Suspension of PPE exportation: Taiwan has manufacturing capacity for N95 equivalent or higher level masks, surgical masks and isolation gowns. In ordinary time, these products are mainly for exportation. Once there is shortage of PPE, Ministry of Economic Affairs and Ministry of Finance can enforce suspension of the exportation of related items. 2. Reuse: Although N95 equivalent or higher level masks, surgical masks or isolation gowns are all disposable, and are in principle not to be reused, when there’s a material shortage, they can be reused with limitation after cleaning and decontamination and under safe situation. For example, before entering isolation wards, wear a surgical mask over N95 mask and an isolation gown over the protection clothes, and when leaving the ward, only the surgical mask and isolation gown need to be replaced. In this way, consumption rate of N95 mask and protection clothes can be slowed down. 6.2.5.2 Speed up Supply 1. Accelerate importation: Obtaining foreign resources with the help of Taiwan’s representative offices aboard. Besides, Ministry of Finance and Ministry of Economic Affairs will assist with rapid custom clearance. 2. Use of expired PPE: PPE stockpiled during the 2003 SARS outbreak still be kept and used if they pass relevant examinations. - 66 - can 3. Alternative: If surgical masks and procedure masks are not available, people with low risk can wear cotton or gauze masks or active carbon masks, because it can still block droplets spread from coughing. 4. Speed up manufacturing: The main restriction factor of mask manufacturing is labor, and no complicated operation technique is required. If supply and demand of mask is unbalanced, soldiers will be deployed to support the mask manufacturing line. 5. Management and delivery of donated PPE: Central epidemic command center will designate a responsible unit to manage donated PPE and their delivery. 6.2.5.3 Stockpile and Release of Masks for Common Need 1. Central government will stockpile 34 million pcs of procedure masks in advance to tackle panic buying. 2. Monitoring mask shortage: (1) Vendor information--In the post-SARS period after 2003, CDC has obtained market information of procedure masks from domestic convenient stores every week. (2) Supply information-- Since 2004, all manufacturers having signed a PPE inter-entity supply contracts with CDC have provided international and domestic market information. (3) Information on needs-- MIS has a “Scheduled Receipts” monitoring function. Once there are “Scheduled Receipts” to regional and higher level hospitals or local health bureaus that have not been checked and acknowledged within 1 month, an inspection and monitoring mechanism will be implemented to understand the reason (4) Market order-- When pandemic shifts to phase A1 or higher, Ministry of - 67 - Economic Affairs and Fair Trade Commission will implement monitoring on PPE market order and price fluctuation. 3. Release timing and price: When the Central Epidemic Command Center decides to release its stockpiled procedure masks to regulate demand and supply after evaluation, logistics and vendor spots in convenient stores set up by Ministry of Economic Affairs will offer a channel for the public to acquire qualified and reasonably priced procedure masks with convenience. The release price and speed will be proposed by Ministry of Economic Affairs and decided by Central Epidemic Command Center. 4. Delivery channels and time limits: Coordination center for mask deployment to convenient stores planned by Ministry of Economic Affairs will send vehicles directly to the central mask storage warehouse to get the masks. It is estimated that within 24 hours after receiving the order of release, delivery will be completed following existing delivery mechanism to more than 8000 convenient stores. Instruction of use is available on small-packaging masks. Barcode is also available to facilitate selling in each retail convenience store. 6.3 Emergency Handling of Abnormality 6.3.1 Back-Up Personnel of PPE Reallocation In response to high attack rate of influenza pandemic, should the current material controllers be unable to carry out their duties, personnel familiar with MIS system and PPW dispatch will be assigned to support . CDC planned to complete simulation training and exercise on PPE dispatch for back-up personnel before the end of October 2006. 6.3.2 Emergency Contact Points Following units should set up emergency contact points to deal with - 68 - emergency in PPE matters promptly. When Central Epidemic Command Center was established and staffed, the contact points will be stationed at command center or participate in dealing with emergency according to the operational procedures of the mechanism and orders of the commander. 1. Fair Trade Commission, Executive Yuan 2. Industrial Development, MOEA 3. Department of Commerce, MOEA 4. Centers for Disease Control, DOH - 69 - 7. Maintaining Health Services 7.1 Rationale During SARS period in 2003, there was no planning at the initial stage on health-care facilities for infectious disease control responsible for receiving and treating patients. Patients sought medical help everywhere, resulting in the spread of SARS virus and nosocomial infection events. Some hospitals were closed, which further caused the collapse of medical system, confusion of public feeling and serious impact to social stability. According to estimations, if the attack rate of influenza pandemic reaches 25%, 3,042,610 citizens in Taiwan will need out-patient service and the number of hospitalization will reach 664,269. Assuming that the pandemic persists for 12 weeks, the demand for hospital bed will reach the peak in the 7th week after onset of pandemic. 98%~124% of hospital beds of all hospitals in the whole country may be occupied, which will greatly push out bed occupancy of other diseases. Hospital beds of existing hospitalized patients cannot be cleared rashly to be used by influenza patients. It will definitely result in collapse of medical system. To prevent the aggregations of large numbers of patients, which will result in cross-infections and cause burden to medical facilities, all local governments should plan in advance to bring the role and function of primary care settings into full play. According to “Article 51 of the Communicable Disease Control Act”, “community disease screening stations” will be set up when necessary. Furthermore, the concepts of “stay home for self care” or “snow-day restriction” can be introduced. Patients should be separated at the early stage of out-patient visits according to pre-planned patient handling principle of human H5N1 or influenza pandemic. According to “Articles 27 and 51 of the Communicable Disease Control Act”, proper planning must be made for the use of hospitals in Medical Network for Prevention and Control of Infectious Diseases, other - 70 - hospitals and large care facilities, taking into consideration patients’ various conditions and different medical resources in each area. Suspected (confirmed) human H5N1 cases will be housed together and treated to prevent paralysis of general medical systems. A Chinese military aphorism goes: “To maintain an army for a thousand days to use it for an hour.” This concept can be extended to manpower back-up strategy for pandemic. A list of manpower back-up should be established, and they should be provided with education, training and drill experience in peacetime. It will be used to transfer and requisite personnel according to “Article 51 of the Communicable Disease Control Act” and “Epidemic Control Materials Requisition Operation Procedures and Compensation Method” at the right time. 7.2 Implementation Strategies There are 23 hospitals in Medical Network for Prevention and Control of Infectious Diseases (around 9,000 beds, including 373 negative pressure isolation beds and 158 general isolation beds). The capacity of the network can only meet a small portion of hospitalization need, so health services for pandemic require implementation of the following measures: 7.2.1 Primary Care Settings In response to influenza pandemic, overall coordination within the medical system should be taken into consideration to bring out maximum function of epidemic control. When pandemic attacks, it should be avoided that a large number of patients crowd into hospitals which results in nosocomial infection. Patients with mild diseases should seek medical help from nearby clinics in priority, so that the role and function of primary clinics can be brought out. - 71 - Local governments should plan in advance the capacity, functions, responding actions of medical facilities and clinics under their control. The role and function of primary care settings should be brought into full play. Primary clinics play the important role of 1st line epidemic control in the entire medical system. Therefore these physicians should enhance their understanding of the epidemic situation and control measures, including reporting, sampling, delivery of samples and patient transfer procedures. Medical staffs and all personnel should properly carry out self-safety protection measures when examining patients. The public should be taught and requested to comply with infection control instructions. The “community disease screening stations” is another option to avoid paralysis of health care system. Local governments should plan of related arrangements of setting up community disease screening stations before Central Epidemic Command Center elevates the pandemic situation to phase B. When pandemic enters phase B, commander will decide whether community disease screening stations will be set up according to epidemic situation at that time. If yes, the set up should be completed within 1 week after announcement of entering phase B and can be activated at anytime. Local governments should plan in advance the responsible medical facilities or large care facilities that can support with set up and transfer of patients. Also medical manpower, medical information and logistic service should be planned. For the reason of availability, it is suggested that places of ordinary public activities be chosen. The number of stations to be set up is decided according to geographical range, number of population and characteristics. Inside the station it should be divided to observation area, waiting area and examination area according to the concept of separation management, so people can seek medical help with order to prevent cross infection. - 72 - 7.2.1.1 Home Treatment The planning for patients with mild diseases seeking medical help is centered on the resources available in primary care settings. Primary physicians not only play the role of medical care provider, but also the role of monitor. With the introduction of the division concept, local health stations will assist the integration of related clinics in the unit of neighborhood. Education to the public will be reinforced to instruct them to go to neighborhood clinics when symptoms occur. It is suggested to separate outpatient visits to fixed visits and mobile visits. For fixed visits, people go to clinics by themselves. First, a phone call is made by the patient to the clinic to register for a visit. Nurses at the clinic will inform the patient of the appointment to limit attending patients to a fixed number and to maintain the space capacity in the clinic. Patients will be classified and divided according to the patient definition formulated by Central Epidemic Command Center. To proceed with patient division promptly, they can be classified according to patient definition and their condition: 1. Non-influenza patients (treatment to be provided according to general medical handling, but to be separated from flu patients) 2. Influenza patients with mild symptoms, subdivided into children, adults and with or without other chronic diseases. Prescription sheets will be sorted by different colors respectively to facilitate rapid prescription, so that patients can go back home as soon as possible and adopt “stay home for self-care” strategy. During patient’s self-care at home, the local health station is responsible for telephone interview. If necessary, the health station will notify local clinic physicians and nurses form a home medical group to go to the patient’s home to perform examination and diagnosis. Or clinic nurses can arrange a route of visit to play the role of “gate-keeper of community health”. Besides, to prevent - 73 - patients’ condition from worsening, clinic physicians must know clearly the back-up hospital of the clinic and maintain smooth communication. A comprehensive information platform is to be established for horizontal and vertical patient transfer. Should patients’ condition worsen, they will be transferred promptly for treatment. 7.2.2 Medical Network for Prevention and Control of Infectious Diseases There are 23 hospitals in the network and 18 supporting hospitals designated by the central government to be responsible for receiving, treating and supporting human H5N1 influenza or pandemic influenza patients with serious symptoms. All other medical facilities and community disease screening stations will arrange transportation of patients fulfilling the pre-defined criteria to the designated hospitals according to the “Principles for Transfer of Suspected Cases of Pandemic Influenza”. The 23 hospitals in the network and other hospitals that are selected for requisition in the future should set up a hospital evacuation plan in response to continuous increase of pandemic patients. Discussion should be made in advance with the regional Command Center and Consulting Committee of the Network for Prevention and Control of Infectious Diseases in the area where the hospital is located. Evacuation plan should be drafted for evacuation of a certain number of contracted beds (including both negative pressure isolation wards and general isolation wards), evacuation by floor (area), and evacuation of the entire hospital. Passage, back-up medical manpower, patient transfer and security should also be considered as a whole. According to Article 51 of the Communicable Disease Control Act, the loss incurred by medical facilities or public places from being designated, dispatched and requisitioned should be compensated properly, the administrative procedures and compensation methods of which will be defined by the - 74 - Department of Health (DOH). If there is loss of profit for hospital caused by receiving patients designated by the regional commander of Medical Network for Prevention and Control of Infectious Diseases, then DOH will subsidize the loss within this period for the difference in profit from the same period last year (excluding medicine and special material fees). In principle, the subsidy is limited to 3 months, starting from the month of receiving patients until the time of DOH announcement of no more epidemic situation. All related subsidy will be paid by CDC. 7.2.3 Large Care Facilities Large care facilities will be activated when the number of hospitalized patients keeps increasing, causing shortage in isolation wards for severe cases despite the execution of hospital requisition and evacuation strategies. Considering the simple and crude equipment of such facilities in comparison to hospitals, subjects received will mainly be hospitalized patients with milder diseases. Local governments should check and plan in advance the related measures such as manpower, capacity, equipment and patient receiving route, etc. The location better be somewhere far from urban areas, with good air ventilation and spacious, with clean water source, compliant with related public health and fire fighting regulations. Commanders of each region will activate large care facilities according to epidemic needs and preparedness plans to receive transferred patients from primary care settings, community disease screening stations or out-patient service of general hospitals. If patients treated in a hospital of the Medical Network for Prevention and Control of Infectious Diseases are recovering, they can also be transferred to large care facilities for subsequent treatment. Should the condition of cases in large care facilities worsen from mild to serious, they will be transferred to a designated hospital - 75 - immediately. Local governments should also complete measures such as facility set-up and control mechanism in the surrounding areas as soon as possible. To strengthen management of large care facilities, after activation decision, responsible staff and organizations should be set up to maintain functioning and related logistic supplies. They will also ensure smooth connection and transportation between large care facilities, medical facilities and community screening stations, so that the patient can receive treatment in the right facility without delay. Large care facilities should be divided and managed, such as administration area (including nurse station) and receiving area. Receiving area should be subdivided to area of pandemic patients and area of patients also having other chronic diseases to be treated separately. For the planning of space and basic equipments of large care facilities, refer to “Medical facilities set up standards”, but negative pressure is not needed and there should be many windows which can be opened for better ventilation. 7.2.4 Handling of Off-Shore Patients When pandemic hits an off-shore island, commander ought to make decisions on how the patients will be treated according to case condition, epidemic situation, hospital capacity, risk of transfer and administration factor, etc. Infected patients can be treated locally, be treated by medical group formed under instruction of commander if necessary or be transferred to the Taiwan main island. All measures in response must be planned in advance by the local government in each off-shore island in view of epidemic development and local capacity according to above mentioned principles, including agreement with civilian airline companies for patient transfer, which is considered one of the important response measures. Drills and practices of all handling measures should be carried out and - 76 - strengthened to familiarize relevant personnel with each response action, to improve their ability to deal with emergency, and to enhance overall efficiency in epidemic prevention. 7.2.5 Deployment of Medical Staffs During pandemic, patients will increase rapidly in every setting, be it a medical facility, large care facility or home treatment. Demand of medical manpower will increase substantially. Local governments should plan in advance the name list of medical manpower to support hospitals of Medical Network for Prevention and Control of Infectious Diseases, large care facilities, home medical treatment and community disease screening stations in response to pandemic. They can be retired staffs of medical facilities, primary clinics and health centers, trained nurse school students, other related manpower, or patients’ relatives (for stay home self-care). They should be informed and be trained before and during the event, and to be dispatched by commanders in pandemic. If cross-regional medical manpower support is needed, commander of the area in need can ask for dispatch from commander of another area after obtaining approval from the Commander in Chief. Bureau of Medical Affairs of DOH or Ministry of National Defense can be invited to form Medical Manpower Deployment Committee when necessary to decide dispatch operation directions. If medical capacity is exceeded, all heath care facilities will adjust the ratio and shifts of medical care workers and adjust current medical operation procedures to make full use of each material and human resource. Central and local governments will encourage people not to seek medical help when not necessary and to perform “stay home self-care”. - 77 - 7.2.6 Compensation for Personnel Deployment and Requisition Local governments must draft preparedness plan in advance in terms of designation or requisition of medical facilities or public venues to setup temporary care facilities. Civilian medical staffs will also be dispatched to assist in care work. Responsible organizations will be subsidized and compensated afterwards according to “Set up and compensation methods of temporary infection medical facilities” and “Epidemic Control Materials Requisition Operation Procedures and Compensation Method”. The central government will offer subsidies when necessary. If the hospitals in Medical Network for Prevention and Control of Infectious Diseases are affected by receiving patients designated by commander in response to epidemic activation, the shortfall in NHI revenue will be subsidized according to “Infectious Disease Prevention and Control Network cooperation contract”. For more information on health services, refer to “Medical Network for Prevention and Control of Infectious Diseases guidelines”(全球資訊網/專業人 仕/應變準備/「感染症防治醫療網」/95 年度第三期感染症防治醫療網/地方 版-95 年度感染症防治醫療網工作指引). - 78 - 8. Response and Execution 8.1 Phase 0 (domestic) and Phase 3 (international) At this time, there’s no possible cases of human H5N1 in our country; there are foreign cases but without efficient and sustained human-to-human transmission. Global cooperation and routine border control will continue in an effort to stop new virus from entering our country. Monitoring and preparedness should be started in our country. New virus strain should be prevented from occurring domestically at the same time. Key points of epidemic control are: 1. Protect the health of poultry and animal husbandry workers (1) Monitoring of animal epidemic is performed to grasp the epidemic situation at any time, in order to promptly prevent animal epidemic from spreading. (2) Strengthen self protection concept of poultry and animal husbandry workers. (3) Reinforcement of seizing smuggling of fowls. (4) Implementation of protection, health management and antiviral prophylaxis to epidemic control staffs when animal epidemic occurs. (5) Enhance hygiene management of wet markets to reduce risks from on-site butchery and selling of live fowls. 2. Border control (1) Educate people to avoid contact with fowls and their excretion when traveling in HPAI-affected areas. (2) Continue to monitor temperature of incoming passengers using infrared thermal monitor. Evaluate infection risks of passengers with abnormal temperature and handle properly. (3) Educate passengers from countries with human H5N1 cases of the - 79 - concept of self monitoring. (4) Perform reporting and sampling according to the reporting criteria announced by DOH. 3. Surveillance (1) Perform reporting and sampling according to the reporting criteria announced by DOH. (2) Once abnormal influenza cases with serious symptoms and influenza-like illness clusters are reported, investigation should be performed. Sampling and testing will be done to clarify the pathogen if necessary. 4. Response of medical system (1)Perform revision, education & training, and inspection of infection control measures (2)The hospitals of Medical Network for Prevention and Control of Infectious Diseases conduct drills on patient management and evacuation. (3)Local government designates large care facilities. 5. Central and local governments have to stockpile antivirals and PPE. Vaccine strategies and stockpile are prepared by central government. 6. Communication: The core task is to strengthen public education of government preparedness, respiratory hygiene and cough etiquette. 8.2 Phase A1 (domestic) and Phase 4 (international) At this time, there are no possible cases of human H5N1 in our country. There are small-scale, localized outbreaks of limited human-to-human transmission aboard. The infection ability of virus increases, but it is not yet easily adaptive to human. In addition to the establishment of central epidemic - 80 - command center, the most important affair is to intensify quarantine measures. Key points of epidemic control are: 1. Border control (1) The initial soft-toned education will switch to compulsory implementation. Passengers and air crew entering from countries in phase 4 will be requested to perform health self-management and be followed up by the local authorities. (2) Passengers entering from affected countries and with suspected human H5N1 influenza symptoms will sent to medical facilities for diagnosis, examination and sampling. (3) Enhance education of dealing with emergency to airport personnel. (4) Preparation of facilities for centralized quarantine of incoming passengers. 2. Surveillance (1) Perform reporting, sampling and testing according to reporting criteria and related regulations announced by DOH, with reinforcement of protection education to staffs performing sampling. (2) Closely monitor international epidemic situation, and enhance communication with affected countries to grasp latest conditions. (3) Strengthen understanding and willingness of case reporting on the part of physicians and managers of surveillance institutions to enhance the timeliness and correctness of every surveillance system. 3. Response of medical systems (1) All health care facilities have to strengthen precaution measures. Local health authorities set up supervision and inspection team to ensure - 81 - infection control measures are carried out. (2) The hospitals of Medical Network for Prevention and Control of Infectious Diseases ensure preparation regarding patient management and hospital evacuation are completed. (3) Local government ensures large care facilities are truly usable. 4. Complete preparedness of antivirals, vaccines and PPE. 5. Communication: Communicate public health messages to overseas Taiwanese living in affected areas, promote public awareness of border quarantine, and educate people about health self-management. 8.3 Phase A1 (domestic) and Phase 5 (international) At this time, there are no possible cases of human H5N1 in our country. However there are large-scale foreign outbreaks of human-to-human transmission. Although the epidemic is localized, virus adaptation to human increases. The most important affair at this stage is to block the new virus from entering into our country. Key points in response are: 1. Border quarantine (1) Issue travel warnings to suggest our people delaying traveling to affected countries if not necessary. (2) Decide the time of activation of centralized quarantine measure on incoming passengers. Passengers from countries with large-scale human-to-human clusters will be arranged to undergo health observation in designated isolation facilities. Should related symptoms occur during observation period, examination and diagnosis will be performed immediately to clarify pathogen and provide proper care. (3) If passengers from affected areas already have fever or related symptoms - 82 - when entering our country, they will be transferred to a hospital immediately. (4) Airline companies must implement health management on air crew from affected areas. (5) If capacity of centralized quarantine facilities for incoming passengers is exceeded, alternatives will be implemented such as home quarantine. (6) Whether flight restriction will be implemented for affected areas will be evaluated of its feasibility and necessity according to epidemic situation before any decision made. 2. Surveillance (1) Perform reporting, sampling and testing according to reporting criteria and related regulations announced by DOH. The latest information regarding clinical symptoms and transmission route will be communicated to physicians and staffs performing sampling. (2) Closely monitor international epidemic situation, and enhance communication with affected countries to grasp the latest conditions. (3) Strengthen understanding and willingness of case reporting on the part of physicians and managers of surveillance institutions to enhance the timeliness and correctness of every surveillance system. 3. Response of medical systems (1)All health care facilities have to strengthen precaution measures. Local health authorities set up supervision and inspection team to ensure infection control measures are carried out. (2)The hospitals of Medical Network for Prevention and Control of Infectious Diseases ensure preparation regarding patient management and hospital evacuation can be operated at ant time. - 83 - (3)Local governments ensure that large care facilities can be operated at ant time. 4. Distribution mechanism of antivirals, vaccines and PPE is ready and awaits orders. 5. Communication: Announce policies of quarantine and isolation. 8.4 Phase A2 (domestic) and Phase 3~5 (international) At this time, single or several possible cases of human H5N1 appear in our country, which might be the result of importation, domestic fowl-to-human transmission, or lab-acquired infection. Global pandemic alert may be at phase 3, 4, or 5. Each epidemic control measure will be the same with phase A1 (domestic) according to different international phase. In addition, the transmission must be blocked to prevent domestic spread from a single case. Key points in response are: Response action in communities with possible H5N1 cases 1. Case investigation (1) Interview with patients about contact history before falling ill to find out possible source of infection. (2) Interview with relatives and other contacts to know about their late travel history and health condition, and to administer antiviral prophylaxis and hygiene education. (3) Reviewing medical records to obtain clinical information. (4) Analyze investigated information to proceed with description of demography, occupation, exposure history, incubation period and transmission mode (to determine if it’s human-to-human transmission, if they are infected by common source). - 84 - 2. Patient management: Treatment in air isolation wards. 3. Enforced hygiene education: Hygiene education will be reinforced aiming at communities with reported patients to make community public understand characteristics of virus, self-protection measures and the correct way to seek medical help, and to avoid panic. Community volunteers will be mobilized to assist when necessary. 4. Strengthened case finding: Search thoroughly within patient’s activity range for suspected cases and perform sampling and testing. Intensifying monitoring function of medical facilities within the affected community. 5. Contact tracing: Asymptomatic contacts will be administered with antiviral prophylaxis. Home isolation must also be implemented and to monitor health condition daily. 6. Strict infection control measures are implemented in community medical facilities. Caregiver of patients wears PPE. 7. Environmental cleaning and disinfection of home 8. If the infectious source is animal, then all animals within a specified range of the source must be culled and sterilized thoroughly. National response action 1. Central Epidemic Command Center monitors epidemic situation daily. The change in domestic pandemic alert level will be announced in appropriate opportunity according to epidemic development. 2. Reinforce education of maintaining hand hygiene, respiratory hygiene and cough etiquette. 3. Strengthen surveillance in the entire country. 4. Combine all monitoring information to evaluate the transmission ability of - 85 - the virus. 5. Decide if further community control measures need to be implemented according to epidemic situation. 6. Report related information to WHO. 8.5 Phase B (domestic) and Phase 5 (international) At this time, small-scale human H5N1 influenza clusters have occurred domestically, and the international pandemic alert may be at phase 5. The most important affair of this period is to prevent small-scale clusters from spreading. Rapid containment should be implemented. Rapid containment If sufficient antivirals are available and food, daily necessities, medical care and emergency services can be normally provided for in the affected community, Central Epidemic Command Center will decide to carry out rapid containment measures. [14] Execution key points are: 1. First stage (1) Active surveillance to monitor change of epidemic situation, collect information on geographical spread, and evaluate necessity to revise containment measures. Also collect recent travel information of patients to evaluate the necessity of enforced surveillance in other areas. (2) Identify the social network and travel history of confirmed patients and their contacts. Contacts should be tracked for at least 7 days. (3) Administer antiviral prophylaxis to contacts. Request them to comply with public health measures at the same time of drug administration. (4) Monitor contacts. Inform them of initial symptoms to pay attention to, - 86 - teach them how to measure temperature and monitor their own health conditions, ask them to report immediately when symptoms occur, and visit or telephone daily to confirm the health condition of contacts. (5) Medical facilities must comply with infection control measures suggested by WHO and DOH. (6) Communication: Reduce panic of people in containment areas, and ask them to comply with government instructions. 2. Second stage (1) People in containment areas implement home isolation to the utmost and prevent unnecessary social contact. (2) Consider compulsory home quarantine in the following situations and provide food, means of communication, psychological support and regular medicine (especially to those with chronic diseases): ①A group of people with previous exposure to virus, for exampl e at home, work places or schools, or in a clearly identified public gathering. ②Exposure occurred in a definite spot or inside a building (such as hospitals or apartment buildings). (3) Stop large gathering activities and consider restriction on public transportation. (4) Dispatch antivirals and administer mass prophylaxis to people within containment range. (5) Communication: Reduce panic of people in containment areas, and ask them to comply with government instructions. - 87 - National response 1. Border control (1) Entry: Maintain “Border control” measures of phase A1 (domestic) and phase 5 (international). (2) Exit: Implement fever screening and health surveillance measures on out-bound passengers. Those with abnormal temperature must hold a diagnosis certificate excluding human H5N1 influenza issued by an airport physician, regional (or higher-level) hospital or testing authority for clearance to travel abroad. 2. Surveillance (1) Set up reporting criteria according to WHO suggestions and communicate related information to physicians immediately. (2) Strengthen understanding and willingness of case reporting on the part of physicians and managers of surveillance institutions to enhance the timeliness and correctness of every surveillance system. 3. Response of medical system (1)All health care facilities have to strengthen precaution measures. Local health authorities set up supervision and inspection team to ensure infection control measures are carried out. (2)The hospitals of Medical Network for Prevention and Control of Infectious Diseases start receiving H5N1 infection cases. (3)Local governments ensure that large care facilities can be operated at ant time. 4. Reinforce education of maintaining hand hygiene, respiratory hygiene and cough etiquette. 5. Decide if further community control measures need to be implemented - 88 - according to epidemic situation. 6. Communication: Reduce panic of all people. 8.6 Phase C (domestic) and Phase 6 (international) Once WHO announce phase 6 of pandemic, phase C will be announced domestically. At the initial stage of this period, if epidemic is still localized in a portion of communities rather than the entire country, then it’s possible to implement rapid containment described in the previous section. If virus spread becomes uncontrollable and coverage expands to the entire country, then the below national measures will be implemented. 1. Border control: Maintain fever screening measures on out-bound passengers in airports. 2. Surveillance: Sampling and investigation of every case will be stopped depending on the epidemic situation, but specimen collection will still be performed for a small portion of patients with symptoms to understand the activity and variation of the virus domestically. 3. Response of medical system (1) All medical facilities thoroughly implement infection control measures. (2) The hospitals of Medical Network for Prevention and Control of Infectious Diseases receive H5N1 infection cases. (3) Local governments have already completed the set up of large care facilities and deployment of personnel, materials and equipments. Once the capacity of hospitals in Medical Network for Prevention and Control of Infectious Diseases in a city or county is exceeded, large care facilities will be activated. 4. Reinforce education of maintaining hand hygiene, respiratory hygiene and - 89 - cough etiquette. 5. Social distance measures will be implemented accordingly: such asschool closure, flexible work, forbiddance of public gatherings and control of public place capacity, etc. 6. Maintain essential services: Maintain supply of water, electricity, energy and communication, etc. In principle, functioning of business will be maintained. However, attention must be paid to infection control measures. 7. Council for Economic Planning and Development observes and evaluates the impact of pandemic on internal and international economies, and draft internal economy recovery plan accordingly. 8. Risk communication. - 90 - 9. Risk Communication 9.1 Correct Consumption of Poultry Products 9.1.1 Rationale There has been no case infected by H5N1 virus through eating poultry or poultry products so far. Human cases all lived in HPAI-affected countries, and most of them had direct contact with ill fowls. However from the results of virus research, safety management of food is still necessary to current affected areas. Ordinary avian flu virus only appears in respiratory and digestive tracts of ill fowls. But HPAI viruses, including H5N1, may probably appear in any part of ill fowls (including meat), and they can still exit in low temperature. Research shows that H5N1 virus in fowl excretion can exits 35 days in 4℃ and 6 days in 37℃. Therefore it cannot be excluded entirely that virus will spread with fowl meat selling and transportation.[15] Especially in some Asian countries, people in remote area are used to breeding fowls and animals at their backyards. They kill, eat and sell fowls and animals, but lack self-protection concepts. And communication of information is not easy. They are regarded as the group with highest risk of H5N1 infection. In industrialized production and marketing system, ill fowls will not leak into food chain because of biological safety control measures. Therefore WHO and World Organization of Animal Health (OIE) all suggest that biological safety control of fowl-breeding industry and fowl goods production and marketing systems be strengthened in countries with H5N1 epidemics.[16] 9.1.2 Implementation Strategies Until now, there has not been a case of local fowl infected with H5N1 virus in Taiwan. Therefore general public don’t have to be panic over consumption of fowl products. General cooking procedure (heating over 70℃ of every part) is - 91 - able to de-activate H5N1 virus. Suggestions of food safety by WHO are: Avoid eating raw poultry meat and eggs, separated preparation of raw and cooked food to prevent contamination, and wash hands frequently during food preparing process, etc. The above suggestions are sufficient in the current stage when traveling to affected areas. Besides, in related symposiums of WHO and OIE, the selling of fowls in wet markets is a higher risk situation being mentioned frequently. Many Asian countries are used to consumption of un-frozen meat, and there are often multiple types of live fowls sold in wet markets. It has been found that part of water fowls (such as ducks) can excrete large quantity of virus but no symptoms appeared. Therefore the risk of virus spread in wet markets cannot be taken lightly. Organizations such WHO also suggest to reinforce hygiene management in wet markets.[16] To protect fowl-related industries in our country and health of the public, we should prepare in advance to prevent future impact even though there is no H5N1 virus invasion domestically. Executive Yuan established “Executive Yuan Special Committee for Forbiddance of Live Fowls Selling and Butchery in Wet Markets” in 2006 to study related measures. It was decided that butchery and selling of live fowls will be forbidden starting in April 2008. After half year of promotion, beginning from 1 October, 2008, those who cull live fowls in and around wet markets and at stores/homes will be fined up to NT$500,000 according to Animal Husbandry Law. Bodies of killed fowls will be confiscated and destroyed. In addition, DOH announced on 7 November 2006 that once the HPAI virus subtype H5 or H7 has been detected in domestic poultry flocks, poultry sale in and around wet markets and at stores/homes will be completely banned. - 92 - 9.2 Strengthening of Respiratory Hygiene/Cough Etiquette 9.2.1 Rationale Respiratory hygiene and cough etiquette can be applied to all patients with respiratory symptoms. There is no systematic research of the effect of covering while coughing and sneezing and cough/sneezing patients wearing masks on control of pathogenic droplets and secretions of respiratory tract. But methods that limit the spread of respiratory droplets should reduce transmission in theory. Wearing masks may be difficult for some patients, therefore the key point should be cough etiquette.[13] 9.2.2 Implementation Strategies In the ”Avian influenza, including influenza A (H5N1), in humans: WHO interim infection control guideline for health care facilities” revised by WHO in 24 April 2006, there are descriptions regarding respiratory hygiene/cough etiquette for health care facilities. These were subsequently reviewed and agreed to at the 9502nd Consulting Committee of Hospital Infection Control in Taiwan on 6 June 2006. They are: 1. Educate all people about respiratory infection symptoms to: (1) Cover nose and mouth with tissue paper when coughing, and then discard the tissue paper into a rubbish can; (2) Wear a mask if bearable; (3) Wash hands after contact with respiratory secretions (using alcohol-based hand rubs or soap and clear water); (4) Keep a distance of at least 1 meter (3 inches) from other people. 2. Promote respiratory hygiene/cough etiquette in medical facilities: (1) Educate all health care providers, patients, relatives, and visitors. Avoid respiratory droplets to prevent spread of influenza virus or other - 93 - respiratory tract virus; (2) Post notices requesting patients and relatives to report respiratory tract symptoms actively and comply with respiratory hygiene/cough etiquette; (3) Post notices requesting people with respiratory tract symptoms to avoid visiting the sick in medical facilities; (4) Provide masks, tissue papers and alcohol-based hand rubs. Locations in which patients gather, such as waiting room, should be provided with priority; (5) Provide hand-washing facilities and resources (such as alcohol-based hand rubs and hand-washing equipment) in general areas. Locations in which patients gather, such as waiting room, should be provided with priority. 9.3 Correct Usage of Respirators and Medical Masks 9.3.1 Rationale Prevention and control of respiratory diseases, such as influenza, should consider the 3 factors of infectious diseases, which are pathogen, transmission pathway and susceptible host. In general, the closer of prevention work to the source of infection, the higher the efficiency and the lower the cost. Therefore public health interventions such as isolation, quarantine, closing of schools and restriction of gatherings should be considered with top priority. Next is installation of local exhaust ventilation, separate patient transport route, and air circulation, etc. When above measures are not entirely effective, measures such as patients wearing masks, respiratory hygiene/cough etiquette or frequent hand-washing may be helpful. Personal respiratory protection is the last line of defense. - 94 - The important modes of transmission of influenza are mainly “droplet infection” and “contact infection”. Even though the probability of “airborne transmission” is not clear, it does have some roles. There are two ways to prevent influenza virus infection: (1) Using filtering materials to trap microbial particles and let clean air pass through to reduce infection risk of wearer, for example respiratory protection gear such as N95 or higher level masks; (2) limit the spread of exhaled droplets to affect outer air, such as medical masks, etc. 9.3.2 Implementation Strategies 9.3.2.1 Usage of Medical Masks Medical masks are originally designed to protect patients in the operating field from contaminants generated by healthcare staffs. It can also be worn by patients in preventing spreading virus-containing droplets or mucus to the environment by coughing, sneezing or talking, in order to reduce the probability of infectious disease spread. Disposable masks can be generally divided to 3 layers: the outermost layer is the protective payer against body fluids, the innermost layer is the supporting layer, the middle layer is the main filtering layer. There are roughly 3 mechanisms of removing particles from the air stream: larger particles are trapped because of inertial impaction, smaller particles are trapped because of diffuse or electrostatic attraction. There exists a range at which no mechanism is dominant. In this range, known as the Most Penetrating Particle Size(MPPS), generally between 0.1~0.3 ㎛, the efficiency of the filtration medium is at its minimum. The diameter of influenza virus is small (0.08~0.12 ㎛). But it usually adheres to cell fragments or droplets with larger diameters when spreading via coughing, sneezing and talking, making it easier to be trapped by masks. - 95 - Most masks are designed with colored outer layer which is liquid-resistant. The correct way to wear a mask is to let the colored layer facing out. If there is a pliable mental nosepiece, wear the mask with nosepiece facing out and on top. Adjust the sheet metal conforming to the shape of nose after wearing it to ensure fit of the mask. 9.3.2.2 Usage of N95 or Higher Level Mask N95 equivalent or higher level masks can block particles of any diameter to the degree of greater than 95%. As for filtering efficiency, N95 equivalent or higher level masks are better than medical masks, which are in turn better than woven cloth masks. In terms of of shape, the entire filtering media of “3-dimentional cup shape” can be utilized. As for “flat” ones, only surface near nostrils can be utilized, not the part that sticks to the cheeks. As a whole, the protection effects of “3-dimentional cup shape” N95 equivalent or higher level masks are superior to “flat” medical masks. Therefore it is suggested that first line medical staffs at high risks use N95 equivalent or higher level respirators. To achieve the goal of protecting wearers, besides high filtering efficiency of mask itself, the fit to the face is an important factor. Without proper fit, the protective function cannot be brought out even with the best protection gear. Therefore, “fit testing” is the first lesson of using respiratory protection gear. From 2004 to 2005, Taiwan CDC cooperated with Institute of Occupational Safety and Health under the Council of Labor Affairs to promote fit test for medical staffs in medical facilities and to choose appropriate personal masks. The fit test needs to be performed at least once a year. When a wearer gains/losses more than 10% of weight, or if there’s significant change in the shape of the face, the test will be performed again. Facing a huge number of medical care providers in the whole country, the fundamental solution is that the department responsible for labor safety and health in each medical facility - 96 - is equipped with “quantitative respirator fit testers” and a complete respiratory protection program to provide timely service. In addition, medical staffs should perform “fit check” every time when wearing a mask to make sure the fit of masks. Cover the mask with both hands and give a puff, and the air should not leak from the cheek part in contact with the mask. 9.3.2.3 Selection of Masks Medical staffs should be aware of risk concept and choose appropriate respiratory protection gear according to extent of environment contamination and exposure probability. N95 equivalent or higher level masks provide general medical staff with sufficient protection. But for medical staffs taking care of infectious patients and/or performing aerosol-generating therapies, such as endotracheal intubation, suctioning, nebulizer treatment, bronchoscopy and cleaning of discard, their risk of infection increases because patients may cough or even throw up when their throat are stimulated. They should choose protection gear of higher standards. Generally, higher level of filtering materials can be used, such as N100 or P100. Half-face or whole-face type of gear which is more fitted should also be considered. Even the more protective and comfortable powered air-purifying particulate respirators (PAPR) can be considered. It provides more complete protection for first line medical staffs and decrease infection probability to the lowest. Disposable masks are suggested not to be used repetitively. During SARS period in Asia, surgical masks were worn over N95 or higher level masks when quantity of masks was not sufficient. Surgical masks were discarded after each use. N95 masks or higher level of respiratory gear were stored in clean and ventilated environment to prolong its usage. A prerequisite is that N95 or higher level masks are well fitted, otherwise flat masks worn outside will increase breathing resistance and probability of leakage. Pay attention to the - 97 - order of wearing and taking off to prevent touching the outer layer of masks which might have already been contaminated. It is best to clean hands by washing hands or sterilization before and after taking off masks. Since the beginning of 2005, many health departments, companies or consumers has started storing every type of epidemic prevention materials. Mask manufacturers also speed up manufacturing to meet orders from everywhere. Taiwan CDC urges medical facilities, public/private organizations and the public everywhere to start storing an appropriate quantity of masks to prepare for epidemic. 9.3.2.4 Clarification of Related Concepts 9.3.2.4.1 Transmission Routes of Influenza Virus We must know the transmission pathway of the disease in order to develop effective pandemic prevention/treatment plan. Even though the virus strain of next pandemic has not appeared, but experts all agree that once pandemic occurs, its transmission pathway should be the same with seasonal flu. There are 3 modes of transmission of seasonal flu: 1. Droplet transmission The conjunctivae or mucous membrane of a susceptible person gets in contact with droplets (usually with diameter >5 ㎛) from virus carrier or infected people. These droplets vary in diameter. Their time of suspension in the air also changes from diameter, sedimentation rate, relative humidity and air currents. Usually particles with diameter >5 ㎛ will fall to the ground within 1 meter (3 inches) after staying in the air for a short period of time. Some researches show that droplet transmission is the major transmission pathway, especially smaller particles. Therefore, measures like respiratory hygiene/cough etiquette plus disposable tissue paper and frequent - 98 - hand-washing of both infected and exposure people are important ways to inhibit the transmission of flu virus. Air-conditioning and ventilation is not important for the prevention of droplet transmission because larger droplets will not float in the air for a long time. 2. Contact transmission Direct (skin-to-skin) or indirect contact (contact with contaminated objects) has been suggested as transmission factors in some studies. So frequent hand-washing using soap and water or alcohol-based hand gel is an important method to limit virus transmission through contact. 3. Aerosol transmission It occurs by dissemination of either through airborne droplet nuclei or small particles containing the infectious agents. Particles with diameter <5 ㎛ will form droplet nuclei after surface moisture vaporizes. The sedimentation rate will slow down and can float with air current for a longer time. If pathogen is still active, it will cause infection after inhalation by healthy people. Divided by 5 ㎛, infection caused by particles >5 ㎛ is called “droplet infection”; infection caused by particles <5 ㎛ is called “airborne transmission”. Although evidence of airborne transmission of flu virus is limited, studies in animals and humans have raised significant concerns that airborne transmission is a potentially important mode of transmission for some infectious agents. Several therapeutic steps that produce aerosol (such as intubations, suctioning, nebulizer treatment and bronchoscopy) could increase the potential for transmission of droplet nuclei. This probability makes consideration of aerosol protection an important part of infection control planning. - 99 - 9.3.2.4.2 Medical Mask Is Not a Respiratory Protection Gear According to CNS defined by Bureau of Standards, Metrology and Inspection under the Ministry of Economic Affairs, the definition of respiratory protection gear is: the general name of personal protection gear worn by individual in harmful environmental air to prevent respiratory harm. Masks that are well known to medical staffs and the public, such as medical masks, surgical masks, procedure masks, active carbon masks and cotton masks, etc, are not the same in protection effects and application range though they all possess nose/mouth coverage. Medical mask is not a kind of respiratory protection gear, and does not require a fit test. Medical masks may be used as barriers against disease transmission by fluids, especially blood, and some large droplets, and they are designed to prevent release to the environment of large droplets generated by the wearer. They are not designed or approved for the purpose of protecting the wearer against entry of infectious aerosolized particles. Medical masks are divided to 2 types: surgical masks or procedure masks. 1. Surgical masks: They are suitable to be used by medical staffs in surgery, laser, isolation, dental department or other medical procedures. The main purpose is to prevent dissemination of microbes, body fluids and biological particles between patients and medical staffs. They can be divided to flat-pleated, duck-billed shape, cone shape or other. The medical mask is secured to the wearer’s head and face by drawstring, ear loop or head ties. Masks with splash visors have an attached antifog-treated plastic shields. There should not be an exhaust valve in medical masks. Surgical masks must pass FDA testing, and are only available in adult sizes. 2. Procedure masks: Flat or duck-billed shape, fastened to the head with ear loops. Splash-proof to some degree, but no testing required. Available in children and adult sizes. - 100 - 9.3.2.4.3 Recommendations on Using Masks for Common People If people develop cold or cough symptoms, a mask should be worn. The microbe will transmit to the environment and to others through droplets of the respiratory tracts. In addition, performing health self-management, resting at home as much as possible, staying away from work or class, not going to public places, washing hands frequently and establishing good respiratory hygiene and cough etiquette are all self-loving and family-loving behaviors that also show respect to others. To avoid transmission through droplets to others, masks can be considered (to prevent expiring droplets). It not only prevents transmission of droplet, it also reduces probability of virus spread through unnecessary hand contact with droplets. Besides, people working in poultry farms better wear a mask to protect themselves. Table 9.1 Comparison of Product Specifications between Respirator and Medical Mask N95or higher level mask (N95 Filtering Facepiece Respirator) Medical Mask Intended use Decrease inhalation of particles Prevent expired droplet <100 ㎛ in the air by wearer contamination by wearer to protect patients undergo surgeries and operating personnel Use limitations Consideration includes One time use prediction of contamination, breakage, deformation, filth, odor, increase of breathing resistance, etc. Can only be used beyond 8 hours proving that - 101 - filtering efficiency will not decrease after prolonged use, and filtering material load <200mg Certificate requirements Passing the most basic level of Reviewed by FDA. qualified dust-proof mask using Marketed with approval 42CFR Part 84 method of NIOSH Filter elements Not to be replaced Not to be replaced Filtering efficiency Protection efficiency of sub-micrometer particles more than 95% Filtering efficiency more than 95% for bacteria Testing aerosol Sodium chloride particles with and particle size Mass Median Aerodynamic Diameter (MMAD) of about 0.3 ㎛ Polystyrene latex sphere test aerosol around 0.1 ㎛ and Staphylococcus aureus filtration test, per America Society for Testing and Materials(ASTM) standard Airflow rate 85 L/min (liters per minute) 28.3 L/min Respiratory flow speed of general middle-intensity worker is around 30 L/min. Therefore 85 L/min is the respiratory flow of high intensity. Test aerosol Must undergo charge Unneutralized test aerosol neutralized test aerosol to achieve Boltzmann balance to prevent particles be attracted to electrostatic filtering material, causing testing error Preconditioning N series of filtering materials must be placed in environment - 102 - No preconditioning of 38℃ and 85% relative humidity for 24 hours before testing, to know the influence of high temperature and humidity environment to filtering materials. Fit test Performed at the first selection None annual fit test required Fit check Required with each use Size Some brands available in 3 sizes Only 1 size generally available. Smaller masks tend to leak more. None Note: 1. N95 mask is the most basic level of qualified dust-proof mask passing 42CFR Part 84 test of NIOSH. Not only trapping efficiency of mask is tested, it also ensures the procedures and quality to a fixed extent. Therefore N95 certification cannot be marked only with same trapping efficiency. The most important is to be certified by US NIOSH (or other certification system). 2. N95 is the most basic level of US dust-proof masks testing standard (42CFR Part 84). In other words, from the protection view, masks if the same or higher levels can also be used. WHO listed all levels N95, N99, N100, R95, R99, R100, P95, P99, P100 within 42CFR Part 84 (dividing dust-proof masks into N, R, P types, with 3 levels of 95%, 99% and >99.7% respectively) and FFP2, FFP3 within EN 149:2001 standard of EU standard as masks of choice in “Guidelines of Infection Control in Response to SARS Hospital Infection”. In addition, qualified masks certified by other countries of the same standards described above are also accepted (N/R/P 95/99/100 or FFP 2/3 or an equivalent national manufacturing standard). - 103 - Table 9.2 Comparison of Functions of Respiratory protection Gear and Masks Masks available in the market Function Respiratory protection gear (all those N95 or higher level masks certified by NIOSH or higher level respiratory protection gear) Block inhalation of particles<100 ㎛ Surgical D2 dust-proof mask Block inhalation of particles<100 ㎛ (surgical N95)* Prevent expired droplet spread to others Prevent blood and possible contagious substance from contaminating the skin, mouth and mucous membrane of the wearer Prevent inhalation of droplets or larger particles. Trap particles of 5 ㎛ or larger in the air to prevent them from entering into mouth/nose Medical mask Prevent expired droplet spread to others Prevent blood and possible contagious substance from contaminating the skin, mouth and mucous membrane of the wearer Prevent inhalation of droplets or larger particles. Trap particles of 5 ㎛ or larger in the air to prevent them from entering into mouth/nose Cotton (or other gauze mask) Prevent expired droplet spread to others and self-made mask *It possesses both characteristics of surgical mask and respiratory protection gear. It can avoid inhalation of particles and prevent expiration of droplets and liquid permeance. It also conforms to regulations of NIOSH and FDA. The full name is Medical mask/N95 filtering facepiece respirators, surgical N95 for short. It is called surgical D2 dust-proof mask in CNS. - 104 - 9.3.2.4.4 Materials of Mask and Filtration Mechanisms of Airborne Particles Respirator and medical mask filters are typically composed of mats of non-woven fibrous materials, such as wool felt, fiberglass paper, or polypropylene. These filtering materials produce circuitous path, using different mechanisms to trap particles to fibers without blocking the open space, so that air can flow through the filter freely. Media used for the filtration of airborne particles do not work by the same principles as those used for the filtration of liquids. Airborne particles cannot block the space between fibers after being trapped to filtering fibers, or it will influence the breathing of wearer. There are 3 major mechanisms to remove particles from the air stream: inertial impaction, diffusion, and electrostatic attraction. Trapping mechanisms are different for large and smaller particles. Larger-diameter particles (>=1 ㎛) will be trapped by inertial impaction. Such particles cannot easily flow around the respirator fibers as air stream because of inertial effect. Particles deviate from the air streamlines and collide with the fibers and may stick to or to be caught in them. Small particles (<0.1 ㎛) are effectively trapped by diffusion. Brownian movement-the process in which the constant motion of oxygen and nitrogen molecules causes collisions between particles-results in an irregular movement or jumping. The complex path followed by the small particles increases the chance that they will collide with the filter fiber and remain there. Another efficient method of capturing both large and small particles from the air-stream is electrostatic attraction. Electrically charged fibers or granules are embedded in the filter to attract particles carrying opposite electric charge. With the same weight, the smaller the particle result in the larger the surface area. The larger the surface area the more easily it is to produce static. Smaller - 105 - particles are more easily trapped and more difficult to remove because of static charge on surfaces. In past times, resin is added to natural wool fibers to retain an electrostatic charge. This addition enhanced the efficiency many times over the basic wool material. However, the efficiency of resin electrostatic filters is degraded when they are exposed to airborne oil mists and other materials that shield the electrostatic charge. Nowadays synthetic fibers are mostly used, such as polypropylene, to effectively resist shielding effect caused by oil mist. Once particles are trapped, they cannot come off easily because of Van der Waals bonding and other reactions. When particles adsorbed the fiber there will be more trapping points and hence trapping efficiency will increase. But when particles are too many block the space, the respiratory resistance will increase. Too many particles will also cause trapped particles to detach from fibers. Hospital wards are in general quite clean, and there is restriction on usage time of respiratory protection gear, so it will not be a problem. Limitation of load will not be a problem for clean wards either. 9.4 Public Seeking Medical Help 9.4.1 Rationale Future pandemic will definitely cause national panic with continuous reports of the media. Even thought the government has stockpiled antivirals and vaccines, the psychological aspect of public should be taken into account. In order to make the public understand every epidemic prevention strategy, there should be education and promotion about how to seek medical help and to prevent infection through all related channels in response to the pandemic threat. The goal is to make people understand how to seek medical help to prevent infection when facing a pandemic. Close communication between the - 106 - government and the public will lead to the satisfactory results that people are confident in, supportive of and cooperative with the authorities, reducing the panic of epidemic outbreak to the lowest extent. The cost to society will thus be relatively low. The key to successful implementation of communication that informs the public about the potential risk of seeking medical care is through effective multi-channel health education and promotion. Government policy is to be delivered and explained in a concise way so that people can obtain correct information timely and make use of it and to cooperate with the authorities. It will prevent social turbulence, block epidemic spread effectively, ensure people’s health and increase government efficiency. Principles guiding the planning of communication strategies that inform the public about the potential risk of seeking medical help are as follows: 1. Early and expertise: Understand the need of the public beforehand, and provide what they need via communication plan. 2. Correct information: Overall planning for delivery of related information to increase public understanding of and cooperation with pandemic control, and to instill in people the correct procedures of seeking medical help to achieve maximum benefit. 3. Message Delivery: Set up a mechanism for promoting and marketing government policy on pandemic control to the public and implement this mechanism effectively. 4. Honest and Opening. Directly explain government strategies through mutual communication with the public to gain trust and to establish professional image of the government. - 107 - 9.4.2 Implementation Strategies 1. Activation Timing At phase B or C (domestic) or phase 6 (international). 2. Work Division Central government: Health promotion in national mass media, cross-departmental coordination and education, provision of correct and timely information. Local governments: Planning of proper execution methods for health promotion and education, and production of local advertisement flyers. Mobilization of systematic promotion channels, like neighborhood heads, police officers and health centers, and of other local promotion channels (information sessions, propaganda vehicles). 3. Core of Execution (1) Procedures and information related to people attending out-patient or in-patient services in hospitals, including community disease screening stations and large care facilities. (2) Contents about the operation of the Medical Network for Prevention and Control of Infectious Diseases and the procedures and principles of seeking medical help. (3) Patients in off-shore island seeking medical help are handled by two possible strategies: “patient doesn’t move and physician moves: local treatment” and “patients transferred to main island for treatment”. - 108 - 4. Execution Principle (1) Plan and communicate in advance. Correct and uniform information (2) Step by step communication to reinforce impression (3) Propaganda channels for mass population, small population groups, specific population segments and individuals (4) Propaganda of information to specific groups (5) Timely evaluation, review and adjustment (6) Ample use of health promotion materials 5. Execution methods and tools (1) Electric media: television, radio, internet (2) Print media: newspapers, posters, LED advertisement, bus advertisement (MRT , coach) (3) PR activities and survey can evaluate people’s needs and thoughts in a quantitative way (4) Dedicated telephone lines for consultation, including CDC’s 1922 hotline, activation of the 177 fever hotline according to situation, and other hotlines (5) Volunteer groups go deep into every level of community or target at important members in the family and group leaders for health education (6) Epidemic control staffs walk out of the office and face the public directly to communicate government strategies (7) Hold national epidemic communication symposiums and seminars aiming at different groups (8) Hold press conferences, information sessions or symposiums routinely to - 109 - release information on related national and international epidemic situations and prevention strategies to let people obtain correct information (9) Personnel of primary administrative organizations and health centers are the main players in conducting health education and promotion. Village heads and neighborhood heads will assist to expand education and propaganda, walking into the public to spread the words. (10) Make national advertising pamphlets and establish communication channels (11) Plan proper locations for out-patient visits, treatment and hospitalization. Make nationally unified identification signs and labels to clearly mark these places (12) At phase C, the news media regulatory authority should handle news, make announcements, conduct media requisition and promote government orders according to Article 5 of Communicable Disease Control Act. (13) Local governments can make use of current message delivery channels, such as “community public health groups”, etc. 6. Execution Contents (1) Correct procedures for seeking out-patient and in-patient services at hospitals, locations of community disease screening stations and large care facilities planned by each local government. (2) The subjects to be received and treated by Infectious Disease Prevention and Control Network and the activation timing of the network according to directions from regional command center, and procedures and principles of patient seeking medical help (3) The principle of medical handling of off-shore island patients is either - 110 - “patient doesn’t move and physician moves” for local treatment or “patients transferred to main island for treatment”. The regional commander activate medical handling according to related factors such as patient condition and off-shore island medical resources Details of above contents are listed in Chart 9.1~9.4 - 111 - Chart 9.1 Planned Procedures of Communication With Patients Seeking Medical Help—Out-Patient visits/Hospitalization Procedures of Plan Central Epidemic Command Center announces phase B Evaluation of setting up community disease screening stations or large care facilities Contents of Propaganda 1. Knowledge of influenza pandemic and the characteristics of the virus 2.Infection control measures that can prevent individuals from getting infected 3.Governments’ policy for pandemic control, especially the planned procedures for seeking medical attention WHO 5 Major Principles Understand the public’s thoughts Decides to set up Set up must be completed within 1 week Information on community disease screening stations and large care facilities, including functions, locations, activation date, how to seek medical care, required IDs and other matters to pay attention to when entering facilities Plan in advance Announce Symptoms appear Seek medical care in community disease screening station or large care facility Health self-management N Y Confirmed as H5N1 or pandemic flu case Treatment and housing arrangement decided by regional commander ASAP Matters pertaining to community disease screening stations and large care facilities: 1.Infection control measures 2.Control of passage, notification of area division 3.Procedures to enter screening station for visit Information transparency Methods 1.Television, radio, internet 2.Symposiums and seminars 3.Poster and advertisement brochures 4.Mass media, bus/subway advertisement, radio, newspaper 1.Propaganda through administers of neighborhood, police, health centers and schools 2.Regional advertisement brochures, posters, booklets ( Indicate detail locations of community screening stations and large care facilities) 3.Community bulletin board, radio, TV or propaganda vehicle 1.Reinforce propaganda and explanation on the 4..Hotlines of health authorities scene by administers of neighborhood, administrative staff, control staff, medical staff, medical facility personnel and volunteers. 2.Explanation of passage control and area division 3.Poster and pamphlet distribution Establish/ Maintain/ Y:1.Patient transfer matters 2.Psychological support to patient and relatives 3.Satisfaction evaluation after recovers N: Health self-management and satisfaction evaluation - 112 - Reconstruct people’s trust 1.Explanation, psychological support & pacification 2.Assistance of transfer by health care workers 3.Distribution of leaflets Chart 9.2 2006 ” Medical Network for Prevention and Control of Infectious Diseases” Flowchart of Activation of Infectious Disease Prevention and Control Hospitals "National Infectious Disease Prevention and Control Hospital" Activation authority: CDC Centers for Disease Control Department of Health “Infectious Disease Prevention and Control Hospital”(23 hospitals) activation authority: CDC Taipei Command Center Northern Command Commander: Center Chang ShangChuan Commander: Commander: Commander: Commander: Commander: CDC 1st branch Lin TsoYan Wang JenHsien Lee JenChi Chuan YinChing Liu YungChing CDC 2nd branch CDC 3rd branch CDC 6th branch CDC 4th branch CDC 5th branch Center General KeeLung Hospital, DOH General TaoYuan Hospital, DOH Taipei Joint Hospital HoPing Region Center Southern Command Center General HuaLien Hospital DOH General MiaoLi Hospital, DOH General NanTao Hospital, DOH General TaiTung Hospital YunLin Region DOH General ChiaYi Hospital, General HsinChu Hospital, DOH General FunYuan Hospital, DOH Region National Taiwan Command Center Kaohsiung City MingShen Hospital General PingTung Hospital, DOH General ShinYin General ChuDon Hospital, DOH Hospital, DOH General KinMen Hospital, DOH - 114 - General ChiShan Hospital, DOH DOH General Changhua Hospital, DOH LianChiang County Hospital Kao-hsiung/PingTung University Hospital Region General YiLan Hospital, DOH Eastern Command General TaiChung Hospital, DOH HsinWu Region, Taipei County Hospital SanChung Middle Command General PengHu Hospital, DOH Chart 9.3 “Medical Network for Prevention and Control of Infectious Diseases” Procedure Flowchart for Transfer of Infected Patients from Off-Shore Area to Main Island for Treatment Off-shore hospital reports patient Report 1. Command centers in related areas evaluate then confirm transfer 2. Fax and send approval to Bureau of Health Off-shore Bureau of Health 1. Initial evaluation of case condition 2. Contact with all organizations for related matters such as patient transfer, receiving and transportation Forward message after receiving response Bureau of Health 衛生局通知 inform 2 2 Inform 1 Response Vice Director Shih Wen-Yi Report Division Director Chiu Jen-Hsiang Inform 3 DOH Center for air transfer evaluation Hsin-Hang Tel:886-2-8195-9119 or 886-2-8911-4119 CEO Tsai Ministry of the Interior Air Service Command Center Tel: 886-2-8911-1100 Executive Yuan National Search and Rescue Command Center Tel: 886-2-8196-6119 Dedicated Fax: 886-2-8196-6740 or 886-2-8196-6741 Response Receiver end Bureau of Health Order announced Response Support 2 Executive Yuan Coast Guard Administration Priority Support 1 Airline company that signed emergency medical transfer contract Departure from main island port Ministry of the Interior Air Service Head Team Departure from main island airport Arrival at off-shore island port Arrival at off-shore island airport Ambulance Sender end Fire Bureau Departure from off-shore island port Departure from off-shore island airport Arrival at receive end port Arrival at receive end airport Receiver end Fire Bureau Ambulance Dispatch vehicle Response Arrival at receiver end infectious disease prevention and control hospital Note: 1.Off-shore areas include Peng-hu county, King-men county, Lian-chiang county, Ping-tung county (Hsiao liu chiu), Tai-nan county Landau village & Green Island village 2. Priority of patient transfer: 1st – Airline company that signed emergency medical transfer contract; 2nd – Air Service Head Team; 3rd – Coast Guard Administration 3. Solid line represents major procedures, dotted line represents response action Chart 9.4 “Patient doesn’t move, physician moves” Operation Procedures for Off-shore Area Suspected or confirmed novel influenza case Coordination and contact Off-shore health bureau Report local epidemic situation, material and human resources and demand evaluation Off-shore infectious hospital or Health station Request manpower support Request manpower support Infectious Disease Prevention Network Command Center Decision of Commander Video diagnosis or local treatment Deployment of medical group No CDC Central Infectious Disease Surveillance System Yes CDC branch director reports to headquarters to confirm off-shore back-up manpower name list. Meet up location/time at main island Assist in buying flight/boat ticket Support in dispatching epidemic materials Inform Support hospital Informs back-up manpower Back-up manpower arrive at meeting spot and go to area in need by plane (boat) Off-shore area Bureau of Health assists in stationing of medical group, coordinating traffic, food and accommodation, as well as continuous epidemic evaluation and reporting back Report back on epidemic handling Stationing in Regional Command Center evaluates the need for continuous support No Medical group 否 - 116 withdraw - 否 Off-shore infectious hospital prepares epidemic control materials and back-up work items Briefing on scale of epidemic and personnel arrangements Yes 9.5 Business and Organization Continuity 9.5.1 Rationale Influenza pandemic will spread cross borders and continents. Not only individual live and health were influenced, economy and society were also impacted to a certain degree. Many employees may not be able to work because of being sick or quarantined. Some ancillary support, such as raw material supply, contractors, logistic supply, transportation and energy resources, may all be influenced. Government’s expectation of business, besides being responsible for protecting employee’s health, is that each business keeps operating during pandemic. Therefore it is suggested that business should perform overall risk evaluation according to individual operation characteristics. Strategies of response should be drafted in advance and conduct drills assuming different scenarios [19]. 9.5.2 Implementation Strategies Feasible preparation suggested to business and organizations at current stage includes: 1. Establish platform for information communication Receive timely and correct pandemic information and make known to employees by network, bulletin board and telephone hotline, etc. Reinforce employees’ recognition and precaution of avian flu in peacetime. During pandemic it can be used as the channel of external communication, to send out messages and to clarify rumor. 2. Build up healthy working environment Sufficient and convenient infection control measures are to be - 117 - provided in work places, such as hand-washing liquid, tissue paper and garbage can. Pay attention to ventilation according to individual environment. During epidemic period, perform cleaning and disinfection of public equipment in the office according to CDC or WHO suggestions. 3. Set up emergency response team A member of high-level management will serve as convener, who combines the company’s environmental safety department, medical personnel, general administrative department, human resources department and public relations department to proceed with planning of matters such as infection control, seeking medical help, environment maintaining and communication, etc. 4. Drafting emergency response plan Each business should not only make use of existing emergency response mechanism but also incorporate 2 major objectives into this mechanism: infection control measures and sustainable operation. The contents of the plan should be made known to internal and external personnel of the business. The plan is suggested to include: (1) Risk assessment of domestic and international epidemic situations and analysis results of financial impact (2) Organizational structure of emergency response (3) Infection control measures: ① Measures that reduce face to face contact, such as teleconference or video conference, working in shifts and flexible working hour system, working at home (consider data backup and information security), reducing shared working space and visitor restriction, etc. - 118 - ②Emergency response measures such as reporting procedures, handling of staff falling ill, channels for seeking medical help, case occurrence in office and cooperation with case investigation. (4) Business continuity program: Identify critical work that must be done during pandemic and evaluate essential material and human resources to maintain the work, train and confirm back-up manpower and goods, establish working procedures of that situation, and backup of system data. (5) Human resources management: Set up guidelines on employee salary, compensation, sick leave and return to work of recovered staffs who are no longer infectious. (6) Communication plan: establish a system for obtaining timely and reliable avian flu or pandemic flu information and proper connection. Decide internal and external emergency communication methods and contact channels during pandemic. Review and revise it routinely. (7) Recovery plan - 119 - 10. Exercises For the preparation of pandemic, WHO not only urges all countries to draft national level preparedness plans but also continues to elaborate on the importance and necessity of exercises to pandemic control. “Exercise Development Guide for Validating Influenza Pandemic Preparedness Plans”[20] was also published in February 2006 to provide reference to all countries. Over recent years in our country, the concept of military exercise has been reinforced and applied to disease control mobilization, for it was a relatively weak link in the overall chain of disease mobilization in the past. We expect that, through various types of of exercises, all preparedness measures can be connected effectively, even with bonus effects. In view of the establishment of disease control systems, Articles 14 to 17 of Communicable Disease Control Act stipulate that central and local governments could activate disease control systems. As for infectious disease prevention, Articles 19 to 27 also bestow all relevant government agencies with the legal authority and responsibility of stockpiling medicines for infectious disease control, early detection of epidemic situations, establishment of medical network and strengthening of health education and promotion, etc. Articles 29 to 31 bestow medical facilities with the legal authority and responsibility of conducting thorough medical consultation and evaluation, taking proper care of patients and practicing proper health management in highly-populated institutions. To thoroughly implement all infectious disease control strategies according to law, strategies, standard procedures and communication platforms should be set up in advance. In view that pandemic attack is unpredictable, exercises should be performed to - 120 - evaluate each preparedness measure. According to Article 18 of Communicable Disease Control Act, should there be a serious infectious disease epidemic or an epidemic caused by biological pathogenic attack in our country, each level of competent authorities should mobilize the entire population for systematic defense and preparedness to implement related disease control measures. 10.1 Rationale 10.1.1 Purpose of Exercise WHO believes that the main purpose of exercise is to practice existing preparedness/battle plan, or to use it to form a project. The comprehensive and broad purposes of exercise defined in our country are: 1. Help the first line personnel, command center or the public to be familiar with existing SOP and each response procedure; 2. Reinforce vertical (between central and local) and horizontal (between central departments) communication channels and negotiate a cooperation mechanism/unspoken consensus; 3. Review possible gaps in every link, to detect problems and to propose resolutions for active improvement. 10.1.2 Exercise Types Exercise is a specialized field. Different types of exercise should be selected based on purpose, budget and requirement of exercise. WHO[20] and the US Federal Emergency Management Agency (FEMA) - 121 - [21] categorize exercises into the following 5 types: 1. Orientation An orientation is the simplest and least costly among the five types of exercises. It takes the form of informal discussion designed to familiarize participants with the structure, role designation and procedures of the preparedness plan, with a focus on issues pertaining to coordination and assignment of responsibilities. 2. Drill A relatively smaller-scale exercise, which aims to develop and maintain skills in a single response procedure. Drills are limited in scope and related procedures should be set up in advance. 3. Table-top Table-top exercise is a process in which officials and/or key staff with emergency management responsibilities are gathered together informally, without tight time constraints, to examine and discuss simulated emergency situations and attempt to resolve problems based on their emergency plans. It can be conducted over a time period from a few hours to a few days. Sometimes “Desk-top” will be misused. 4. Functional The completeness is only second to Full-scale (see next item for description), and is more challenging than Table-top. Participants react in accordance with a series of simulated events according to the individual roles they play. The emphasis is on understanding the interaction between important strategies during an emergency event. It is different from a table-top exercise in three ways: first, it is interactive between roles; second, it is conducted under time constraints that would be similar to, or often more challenging than a - 122 - real event; third, it is usually conducted in a facility designated for coordination and management of a real event, so the available tools and technologies can be used and evaluated. A single or multiple emergency situations can be exercised, in order to implement strategies, role, mobilization and division of work, response ability and emergency response procedures. Many resources are required to ensure maximum benefit of functional exercise. There are three ways to perform functional exercise, including planned ruling, free ruling or half free (half planned) ruling[22]. In general, a “reference document” is to be prepared, which is the solution to the exercise situation. 5. Full-scale A full-scale exercise is the most complete and usually the largest type of exercise. It resembles actual emergency, including actual deployment of the resources required to demonstrate coordination and response capabilities in as realistic a setting as possible without putting the safety of the public and staff at risk. A full-scale exercise focuses on the operational ability in emergency events. 10.1.3 How to Plan An Exercise Following above purpose and types of exercise, WHO suggested[30] that in the early stage of planning, following 4 prerequisites should be thought over: 1. Is there a policy-level committee to organize and (or) participate in the exercise? “Policy-level” usually refers to administrators, whose role is to - 123 - lead and provides direction. In central government, it is usually director of a department or a high-level executive official in administrative organizations. In local or non-governmental organizations, it is usually a high-level official or president of board of committee (board of directors/board of committee). 2. What are the intended scope and objectives of the exercise? Scope and objectives are linked. “Scope” is decided by the range of the plan and participation number of organizations. The larger the scope, the more objectives can be included, but realistic limits should be established. 3. Who will coordinate the exercise? One organization or individual must be responsible for coordinating the exercise. In the case of either an orientation or drill, the coordinating agency usually will be the principal author/owner of the plan. In all other types of exercises, the coordinator may be from outside the agency that prepared the plan. In “full-scale exercise”, it is common to have a coordination team with members drawn from all of the significant participating agencies. 4. Who should participate in the exercise? All plan-related organizations should participate in the exercise. In the case of table-top exercise, it is common to allow a number of observers who have a professional, operational or policy interest in the outcome, but care should be taken to ensure that the presence of too many observers does not interfere with the conduct of the exercise. - 124 - Source of funds (or sponsor) should also be considered. Commander of the exercise should form “design team” to perform exercise planning. The 4 major elements of exercise planning, namely scenario, control plan, exercise plan and evaluation plan, should be developed. They are described briefly as follows: 1. Scenario: It should be close to actual situation based on a series of hypotheses and participants are to respond to the scenario according to decided actions and strategies 2. Control plan (or called general plan): describes how exercise will be carried out 3. Exercise plan (or called guiding plan): provides a higher-level blueprint for the overall structure of the exercise 4. Evaluation plan (or judgment plan): describes how exercise will be evaluated and what are the evaluation tools 10.1.4 Organizational Structure of Exercise The organizational structure of exercise is approximately as below chart [22]: Exercise Development Team Exercise Director Assistant Exercise Director Exercise Control Team Participant Support Team - 125 - Exercise Evaluation Team Exercise Force Related roles and assignments are described below: 1.Exercise Development Team: “Exercise Director” is assigned to serve as the highest commander in the exercise. Assistant Exercise Director is assigned to assist, to act as deputy and to supervise ruling work. In small-scale exercises, Exercise Director can serve concurrently as director of judgment, who is responsible for planning, guiding, controlling and reviewing. (1) Planning: Exercise conducted and led by a single (top-tier) authority, such as Executive Yuan, DOH, CDC, county/city Bureau of Health or a hospital, etc, must include control plan (general plan), exercise plan (guiding plan), evaluation plan (judgment plan). Exercise conducted and led by a second-tier authority, such as Bureau of Pharmaceutical Affairs under DOH, the Fourth Division of CDC, Section of Disease Control at a County/City Health Bureau or infection control team at a hospital, can be presented in the manner of teaching plan, including a teaching outline, exercise composition chart and guiding plan chart. (2) Guiding and controlling: including announcing exercise orders, assigning judgment officer and guiding the proceeding of exercise,etc. (3) Reviewing: Review of performance of participants, and suggestions on reward and punishment will be sent to human resources unit to be processed accordingly. 2.Exercise Plan Control Team: Draft all plans of the exercise and draw up/control all proceeding of the exercise 3.Participant Support Team: Provide service/business matters during the course of the exercise 4.Exercise Evaluation Team: Perform the role of guiding and judgment; - 126 - draft and compose ruling plan according to objectives, type, mode, and extent of control; and assign evaluators according to professional specialty. It should be avoided to assign evaluators from participating units in the exercise. In “drill exercise”, the evaluation method of divisional supervision can be adopted. On-the-scene evaluators can be assigned at the spot according to professional specialty. In “functional exercise”, centralized supervision can be adopted. 5. Exercise Corps: All participants. 10.1.5 Affairs to be Managed After Exercise[20,22] 1. If the exercise is more than 1 day, “On-the-day review meeting” should be held daily. Participants report what they have learned and review successes and shortcomings. 2. “Evaluation meeting” should be held within 2 weeks after exercise completion 3. Every question at the review meeting should be noted and answered to in a report, which will be divided and submitted to related organizations to process accordingly. The processing outcomes should be reported. 4. After exercise completion, exercise records should be cataloged as a source of history, including scenario, all plans, judgment (review) report and other documents which are sufficient to record actual events. 10.2 Implementation Strategies As mentioned in the 1st chapter of this plan, pandemic is considered “non-traditional” security threat by APEC, Journal of US Foreign Affairs - 127 - and our country. On the basis of expansion of national security concept, DOH has given more weight to and deepened the scope of “exercise” with regard to “influenza pandemic”, including participation in international exercises, conduct central-level vertical/horizontal exercises and supervise local governments in conducting different types of exercises. In order to conduct more specialized and systematic exercises, DOH asked National Defense University to provide guidance with their specialty on military exercise. “Seminar on Military Exercise Application –Taking influenza pandemic as an example” was held in July 2006, with the planning of courses focusing on “managerial positions” and “staff positions”, in order to establish mutual language and a unified direction with regards to exercise type, exercise planning, scenario composition and review method, etc. 10.2.1 International Exercise The APEC “Pandemic Response Exercise” was organized and held in Australia on 8 June 2006. Our country participated in this exercise simultaneously with other countries. The exercise started at 9am Taipei time. Eight member bodies participated as primary players, including our country, Japan, China, Indonesia, Malaysia, Chile, Korea and Vietnam. Twelve other member bodies, including the US and Canada, acted as secondary players. WHO, OIE and UN Food and Agriculture Organization acted as observers. The exercise response group of our country was formed by related organizations such as DOH, CDC Ministry of Foreign Affairs, Bureau of Animal and Plant Health Inspection and Quarantine under Council of Agriculture, Disaster Prevention and Rescue Committee. Chen Zai-Ching, Vice-Minister of - 128 - DOH, acted as exercise commander of our country, and relevant personnel were stationed at “National Health Command Center” on the 7th floor of CDC headquarters after exercise started. During this exercise, information was delivered between member bodies via telephone, fax or email. The main objective is to test whether the communication network and information sharing channels were smooth, ability of member bodies to deal with emergency and regional coordination/cooperation mechanism. During the exercise, our country received 6 scenarios brought up by exercise coordination center: 1. WHO pandemic alert upgraded to phase 5; 2. Destruction of PPE stockpile due for delivery to Taiwan in an overseas factory by fire; 3. Types of assistance Taiwan could offer to affected countries; 4. Request from the Canadian government to check on the wellbeing of their back-packers; 5. Taiwan’s inclination to participate in the upcoming APEC Youth Football Tournament held in Danang, Vietnam; 6. Request for medical support from a cruise ship with suspected pandemic flu cases on board. After receiving these scenarios, all related units immediately proposed strategies and solutions in response to these scenarios. The strategies and solutions were reported to the Commander. After approval by Commander, our reply was reported back to the Exercise Coordination Center within designated time. The exercise terminated at 3pm. In the future, our country will continue to strive for participation in related international exercises. - 129 - 10.2.2 Central-Level Exercise The central-level exercises already held or will be held by DOH for influenza pandemic are as follows: 1. 7 July 2005: Functional exercise for pandemic phase A1~A2; Exercise items are: (1) Organizational operation in pandemic phase A1; (2) Organizational operation in pandemic phase A2; (3) Procedures for delivery of samples collected from in-bound passengers from infected areas; (4) Investigation of novel influenza patients being picked out for epidemic investigation; (5) Transfer procedures for suspected novel influenza patients; (6) Handling operation when poultry/animal husbandry workers develop suspected symptoms; 2. 27 Dec 2005: Functional exercise of novel influenza in phase B/C; Exercise items are: (1) Quarantine and centralized quarantine measures on incoming passengers at CKS airport; (2) Preparation aiming at suspension of international flights and mini-three link traffic with China; (3) Preparation measures of all departments under Executive Yuan in response to pandemic phase B; (4) Actions of all competent authorities in response to novel influenza cluster events; (5) Preparation measures of all departments under Executive Yuan in - 130 - response to phase C pandemic. 3. 6 April 2006: Drill on airport management of incoming passengers during pandemic; Exercise items are: (1) Central activation of domestic phase A1 epidemic. It is announced that centralized quarantine measure will be implemented for incoming passengers from affected areas. A press conference is be held to inform the public; (2) All related units of CKS airport convene a meeting on centralized quarantine measure in response to pandemic phase A1. Units related to entry promptly arrange and set up special places to perform entry customs clearance. Once flight arrives, ground staffs will be ready to activate special entry clearance services. (3) During check-in an boarding, staffs of airline companies stationed aboard inform passengers of Taiwan’s centralized quarantine measure on in-bound travelers; (4) One hour before the landing of a plane, the captain reports to control tower that there are 2 passengers with fever on board. After landing, the flight purser announces special cautions related to centralized quarantine before passengers disembark; (5) Immediate handling and following management of passengers detected to have abnormal temperature by infrared thermal scanners at the airport (including recheck of temperature and diagnosis by an epidemic control physician); (6) Issuing “centralized quarantine notice” to passengers with normal temperature; (7) Handling non-compliance of passengers to get on the ambulance; - 131 - (8) When there are insufficient ambulances at the airport, contact is made to request dispatch of other ambulances. When the capacity of the designated hospital for patient transfer from the airport is exceeded, patients will be sent to other hospitals; (9) Passengers go through ID check and luggage check at the customs; (10) Joint handling of smuggled birds by quarantine staffs from the airport customs office, Bureau of Animal and Plant Health Inspection and Quarantine under the COA and CDC; (11) After completing all custom procedures, passengers are sent to centralized quarantine facilities by a vehicle. ; (12) Personnel take off their gear and clean the scene. 4. At next stage, exercises will be held to test the medical system’s response capabilities; Exercise items are: (1) A hospital reports one inpatient with pneumonia of unknown cause. Bureau of Health performs case investigation. Sampling of the case confirms H5N1 influenza infection; (2) In response to the first confirmed domestic case of H5N1 influenza, the “Central Epidemic Command Center” is launched; (3) H5N1 influenza cases are reported in succession in central Taiwan and first wave of epidemic begins; (4) Suspected H5N1 influenza cases are reported in succession everywhere in Taiwan; (5) Due to rapid increase in reported cases, commander announces suspension of sampling and case investigation in order to control the response capacity and to adjust the control emphasis. Sections 1, 2 and 4 pertain to functional exercise, while section 3 pertains to drill exercise. Central-level exercises are centered on - 132 - information collection (information flow), policy-making (strategy decision) and procedures of passing down orders (chain of command) by Central Epidemic Command Center during a pandemic. Therefore, they are mainly in the form of orientation exercises, table-top exercises and functional exercises are usually used. In section 3 “Exercise for airport management of in-bound passengers during pandemic,” a drill exercise was used. Because in national epidemic control strategies, border control is an important and universal measure. Therefore it is not appropriate to change the location of the procedures. As for exercise on disease control, there has not yet been a full-scale exercise domestically or internationally. Full-scale exercise is similar to the occasional air defense exercise or wan-an exercise, which is conducted in actual and open territory. Once people appear in the exercise scene, they must cooperate with the exercise, and are regarded as participants. The appropriateness and execution methods of full-scale exercise in disease prevention/treatment are to be evaluated. During exercise, evaluators are assigned. In drill exercises, additional “on-the-scene evaluators” will be assigned. The invitation of evaluators should be based on their professional specialties, and complete exercise data and evaluation criteria should be provided in advance. On the day of exercise, evaluators/on-the-scene evaluators will comment and review, and record according to the facts. All participating units, evaluators and on-the-scene evaluators will be invited on another day after the exercise to hold a joint “evaluation meeting”. All reviewed matters will be forwarded to related units to proceed with correction. That way, the actual effect of exercises can be achieved. - 133 - 10.2.3 Local-level Exercise Local governments should perform orientation, table-top or functional exercises following the example of central government according to needs. The exercise should focus on information collection (information flow), policy-making process (decision flow) and order receive/announce procedures (chain of command) by “local response center” during pandemic. All offices and sections of local government are at the first line of epidemic control, therefore control procedures of all diseases and the single measure “drill exercises” should be strengthened. The main objective is to familiarize 1st line personnel with all SOP and execution methods so that they are able to response swiftly once an epidemic occurs. Each local government differs in geographical environment, population, extent of urbanization, climate, culture, funds and organizational structure, and even central strategies still need to be adjusted accordingly. Therefore, a local government should establish its own exclusive “SOP” and “checklist” according to relevant laws, regulations and strategies to be followed by the first line personnel and to serve as the basis of “exercise plan” and “evaluation plan”. Drills that local governments should execute in response to the pandemic threat can be generalized in 10 categories. Other special drills should be proceeded actively by local governments: 1. Response center: Information flow, policy-making process and chain of command; 2. Case management: Reporting, surveillance, delivery and testing of samples and case investigation procedures (including monitoring of information system); - 134 - 3. Resource management: Local epidemic prevention resources (including: human resources, logistic service goods), stockpiles, reserves, delivery and management (including monitoring of information system), etc; 4. Infection control: Management of Designated Infectious Disease Prevention and Control Hospitals in terms of coordination of hospital beds, (including monitoring of information system), nosocomial infection control management, human resources and institutional capacity; 5. Patient delivery mechanism: Procedures of case transfer and related protection and disinfection; 6. Communication plan: vertical (with central authorities), horizontal (across all bureaus and departments, cross county/city governments) and communication with public. Proper health education and media interaction; 7. Management of large number of cases: Centralized quarantine of passengers returning to Taiwan from affected areas and software/hardware planning for large care facilities; 8. Community restriction: Various community restriction measures and society stabilizing strategies; 9. Handling of remains: handling procedures of the dead cases and capacity management; 10.Recovery of society: Reconstruction in the recovery phase of the pandemic. - 135 - Appendix - 136 - Appendix 1. Characteristics of Avian Influenza A(H5N1) Virus 1. Virology Influenza virus is an RNA virus belongs to the Orthomyxoviridae family. It has sheath and is sensitive to ether. In the sheath there are 2 types of glycoprotein: hemagglutinin(HA) and neuraminidase(NA). HA enters cells of host by binding to sallic acid receptor in cell surface of host. It is the most important virulent factor of virus’ invasion into cells. It is also the most important antigen in virus antibody neutralization test, and can be used to produce vaccine. NA is the 2nd most important antigen in flu virus neutralization test. Its action is to break sallic acid connecting virus and host cells, which helps virus granule enter host cells. It is an important target protein for anti-virals. The 3rd glycoprotein in virus surface is M2 protein, which is only present in the surface of a few influenza A virus. It is mainly ion channel protein used to control the ph value of virus. It also affects the initial phase of virus replication, therefore it is also the target protein of some anti-virals. Gene hereditary substance is a reverse RNA. It is divided into 8 segments. Important virus genes of each segment are listed below: Segment Gene Gene function 1 PBa RNA transcriptase 2 PB1, PB2 RNA transcriptase 3 PA RNA transcriptase 4 HA Hemagglutinin (bind to sallic acid) 5 NP Constructional protein 6 NA Neuraminidase (cut sallic acid) 7 M1 Matrix protein (help virus recombination) - 137 - 8 M2 Non-structure protein (ion channel in virus surface) NS1 Non-constructional protein NS2 Non-constructional protein Influenza is divided to type A, B, C according to N, P and M protein. Up to now, all avian flu is caused by type A influenza virus. Virus A is subdivided by HA and NA. There are currently 16 types of HA (H1~H16), and 9 types of NA (N1~N9). The late outbreak of avian flu is confirmed to be caused by H5N1 type. 2. Pandemic Caused by Avian Flu Virus HA gene of avian influenza A derived antigen has never caused epidemic in human. Therefore, human immune system lacks immune function to virus with this kind of antigen. Once virus can transmit within human, there will definitely be a rapid spreading and will cause global pandemic. There were 3 similar influenza pandemics in the 20 th century, in 1918, 1957 and 1968 respectively, which killed millions of people globally. The pandemic in 1957 and 1968 were originated from south Asia. Its genotype is between avian flu virus and human flu virus. The virus strain serotype of avian flu pandemic in 1957 was virus H2N2. It is the hybrid of avian flu virus in wild ducks and human H1N1 flu virus. “Hong Kong influenza virus” pandemic in 1968 was caused by new H3N2 virus from hybridation of H and PB1 gene in duck flu and H2N2 human flu virus. It resulted in human-to-human pandemic. As for the “Spanish flu” outbreak in 1918, human didn’t have immunity against its H1N1 virus and resulted in global pandemic. It is estimated that 1 million people died because of this flu. According to research, the reason for 1918 flu outbreak may be the same with outbreaks in 1957 and 1968. - 138 - 3. History of Human Infection by H5N1 In recent years, highly pathogenic avian flu virus is gradually able to infect human, and cause serious or even deadly symptoms. H5N1avian flu virus has attacked many Asian countries. According to previous human infection cases, H5N1 virus is limited to bird-to-human. But in southern Asia, living space of human and domestic animals (such as pigs) are very close. These kinds of animals are probable media for avian flu mutation of human adaptivity. According to an essay that H5N1 virus has been found in pigs. Low-pathogenic avian flu antibody is also detected in many pigs. These conditions are similar to those of 1957 and 1968 avian flu pandemic outbreaks, which should be paid attension to. 4. Clinical Symptoms of Human Infected with H5N1 Flu Virus According to analysis of available case data, latent period of human infected with H5N1 virus is mostly 2 to 4 days. In some rare cases, it can be as long as 8 days. Infection characteristics are serious flu symptoms. Its clinical manifestation includes fever, cough, short breath and pneumonia observed radioactive imaging. Especially that pulmonary damage can still be observed through radioactive imaging several months after H5N1 infection. In addition to respiratory symptoms, a large portion of people presents digestive system symptoms. These symptoms are similar to symptoms of some children infected with influenza, such as vomit, diarrhea and abdominal pain, etc. H5N1 symptoms are not localized to acute respiratory tract syndrome, some cases also present renal failure, multi organ failure, coma and death. H5N1 virus can be separated from throat swab, feces, blood and cerebrospinal fluid. Therefore it can be concluded that H5N1 infects - 139 - organs of the whole body extensively. Until now, it is still rare for H5N1 to invade central nervous system. Host genetic expression plays a very important role in symptom manifestation of disease. In addition, patients infected with H5N1 virus will present a special immuno-phenomenon. Large quantity of chemokines and cytokines will be present inside patient’s body. These hormones will attract large quantity of white cells to lungs, causing self-immune cells to aggregate in lungs. Phagocytosis and cytotoxic of lung cells infected with virus will cause cell lung necrosis and function loss. This autoimmune reaction is not localized to lung; others include fibrosis of small intestine tissue, necrosis of liver cell and some blood diseases. It is suggested that some local ill tissues be tested of virus RNA. 5. H5N1 Flu Virus Laboratory Diagnosis 5.1. Culture of virus Avian flu virus can be cultured by egg-based technique or by cell strain infection. Cells used are kidney cells of rhesus and Madin Darby dogs. The difference from normal cold virus is that highly pathogenic avian flu virus cell culture does not require addition of pancreatin to facilitate virus replication. Labs of highly pathogenic virus culture should better be biological grade 3 or higher. Samples better be collected by throat swab and respiratory tract gargle. H5N1 virus can be separated from other samples, including serum, cerebrospinal fluid and large intestine smear. 5.2. Detection of antigen Current influenza virus A detection methods are direct fluorescent immunoassay and immunochromatography. However the sensitivity of - 140 - these test are low. Antigenic tests to H5N1 virus are still under research. 5.3. Reverse transcriptase polymerase chain reaction (RT-PCR) It is currently the method with more H5N1 specificity and sensitivity. The gene of H5N1 is a reverse RNA, therefore reverse transcription is used to transcript RNA reversely to cDNA. Polymerase chain reaction will magnify specific segments of H5N1 as screening. 5.4. Serological detection When there is an avian flu outbreak, detection of H5N1-specific antibody becomes very important. Blood aggregation test is the standard testing method to detect influenza antibody. However this test still cannot be used in detection of H5N1 antibody in serum. A possible reason is that H5N1 virus is not able to induce large fluid immuno reaction, causing insufficient H5N1-specific antibodies in mammals and low sensitivity in blood aggregation test. Sensitivity is higher when using ELISA to detect neutralized antibodies in serum directly, also with sufficient specificity. Therefore it is a good choice to detect neutralized antibodies directly. 6. Treatment and Prevention 6.1. Antiviral treatment At present, there are 2 types of anti-virals: M2 inhibitors and neuraminidase inhibitors. M2 inhibitors are launched earlier and are cheaper. But resistance is more frequent according to using experience. In addition, it is not known whether it’s safe to pregnant women. Another major point is that H5N1 has been found to be resistant to M2 inhibitors. Other type of new medicine is neuraminidase inhibitors, such as Oseltamivir and Zanamivir. This type of medicine is safer, and with less - 141 - concern of resistance. However it is more expensive and its capacity is limited. Oseltamivir is only effectively when used at early stages of symptom occurrence. Zanamivir is a spray, and is only effective in respiratory tract infection part. Therefore, it is suggested that these 2 neuraminidase inhibitors be used within 48 hours to achieve better therapeutic effect. 6.2 Infection control and prevention When fowls are infected with avian flu, large amount of virus will appear in their excrement and secretions. These contaminants will spread extensively within the environment, such as water, dust, soil, bird cages and some utensils. Avian flu virus can exist in soil and water from weeks to months. The lower the temperature, the longer it survives. Flu virus can be found in most tissues and organs of the bird infected by highly pathogenic avian flu, even in the egg it laid. Therefore, it is highly probable that highly pathogenic avian flu virus be transmitted to human through bird-to-human mode by direct/indirect contact with or consuming of these bird excrement or products. To prevent avian flu from evolving to human adaptive, isolation measure should be implemented to patients infected by avian flu. Their excretion and secretion should also be collected and be sterilized. In addition, try not to get into contact with wild birds as much as possible. Wear a surgical mask to prevent infection if necessary. Nueraminidase inhibitors can be administered to close contacts with avian flu patients and medical staffs as preventive medication to prevent its spread in hospitals or densely populated areas. - 142 - 6.3. Vaccine The human influenza vaccine is produced by culturing highly pathogenic avian flu virus in eggs. This procedure is suggested to be operated in labs with biological safety grade 3 or higher. The H5 antigen is presented in large quantity by genetic engineering, or by using DNA vaccine to make H5 gene a target gene. However DNA vaccine is not effective to some heterogeneous H5N1 in mice study. 7. The Use of Disinfectant In the guidelines for H5N1 infection control in medical facilities announced by WHO in 24 April 2006, ethanol and bleaching agents (sodium hypochlorite) were suggested to use. Ethanol effectively inhibits activity of flu virus. Ethanol of 70% concentration is an effective and extensive disinfectant, which is often used to sterilize a small range of surface. Bleaching agent is a very potent and effective disinfectant. Its active ingredient, sodium hypochlorite, kills bacteria, mold and viruses, including influenza virus. Diluted home bleaching solution is effective in different contact time (10minutes~60minutes). - 143 - Appendix 2. Estimation on Health Impact of Influenza Pandemic Senario 1 Influenza Pandemic Impact Hospital Admission / Weeks 1 Weekly admission 4,489 2 3 ICU Capacity 5 6 7 8 9 10 7,482 11,223 14,215 14,215 11,223 7,482 4,489 Peak admission/day Hospital Capacity 4 2,215 2,215 # of flu patients in hospital 4,489 % of hospital capacity used 6% 10% # of flu patients in ICU 673 1,428 2,193 2,897 3,135 3,050 2,423 1,673 % of ICU capacity used 10% 22% 34% 37% 26% ventilators 337 714 1,096 1,448 1,567 1,525 1,212 837 % usage of ventilator 3% 7% 11% 14% 8% # of deaths from flu 838 1,396 2,095 2,653 2,653 2,095 1,396 # of flu deaths in hospital 586 7,482 11,223 14,215 14,935 13,749 10,640 7,015 15% 19% 44% 20% 48% 18% 47% 14% 9% # of flu patients on Ventilator Capacity Deaths - 144 - 977 16% 15% 12% 1,466 1,857 1,857 1,466 977 838 586 Senario 2 Influenza Pandemic Impact / Weeks 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Hospital Weekly admission Admission Peak admission/day Hospital # of flu patients in hospital 748 Capacity % of hospital capacity used 1% 4% 7% 10% 13% 15% 15% 14% ICU # of flu patients in ICU 112 500 989 1,479 1,968 2,346 2,475 2,428 2,080 1,631 1,153 675 Capacity % of ICU capacity used 2% 8% 15% 23% 30% 36% 38% 37% 32% 25% 18% 10% Ventilator # of flu patients on ventilators 56 250 495 739 984 1,173 1,237 1,214 1,040 816 576 337 Capacity 1% 2% 5% 7% 10% 12% 12% 12% 8% 6% 3% # of deaths from flu 140 559 977 1,396 1,815 2,095 2,095 1,815 1,396 977 559 140 # of flu deaths in hospital 98 391 684 977 1,271 1,466 1,466 1,271 684 391 98 Deaths % usage of ventilator 748 2,993 5,237 7,482 9,726 11,223 11,223 1,749 2,993 5,237 7,482 - 145 - 9,726 7,482 5,237 2,993 748 1,749 9,726 11,223 11,791 10,989 9,376 7,132 4,887 2,643 12% 10% 9% 6% 977 3% The above estimation was simulated under 25% attack rate by FluSurge. Variables Age Group Values References 0-17 yrs 5,338,586 Stastics from Ministry of 18-64 yrs 15,199,201 the Interior in January, + 65 yrs 2,158,562 2005 Total staffed beds 76,074 2003 Health Statistics Staffed ICU beds 6,526 2003 Health Statistics Total number of 10,000 Estimated ventilators Duration Senario 1: 8 weeks Senario 2: 12 weeks - 146 - Estimated Appendix 3. Excerpt of Presidential Instructions to High-level National Security Meetings First High-level National Security Meeting on ”Response Strategies to Possible Invasion of Avian Flu” 19 August 2005 Even though avian flu is not the same as SARS, we should bear in mind the lessons learned from our previous mistakes. Below is a summary of some major deficiencies that should not have occurred during course of fighting SARS for everyone’s reference. They will be further reviewed to see if previous lessons have indeed been learned to assist relevant preparations in response to the pandemic threat. 1. Lacking a communication mechanism between central and local governments, which led to inconsistencies in the pace of fighting SARS and in government announcements, and sometimes even to hostility between relevant authorities due to unprofessional factors. 2. Incomplete patient reporting and control system. Individual patients moved from one medical facility to another to seek care, causing rapid spread of the epidemic. In addition, patients tended to seek medical help in emergency rooms in teaching hospitals or medical facilities of equivalent status. If nosocomial infections occurred in these hospitals, the worse case scenario would force hospital closure and result in paralysis of the whole medical system. 3. Screening of confirmed cases took too much time. The number of suspected patients was always high. Related isolation and control measures were difficult to implement, and resulted in unnecessary panic. 4. Insufficient training to frontline medical staffs in self protection and - 147 - lack of strict compliance with related SOP, which resulted in injuries and deaths of medical staffs. 5. Severe insufficiency of epidemic prevention equipment and materials resulted in worries and dissatisfaction of medical staffs and the public. Under provocation and manipulation by special interest groups, there were distrust and severe criticisms of the government. 6. Relevant epidemic prevention and medical information was not delivered timely and explained properly. Matters that required public cooperation and health self-management were not communicated concisely. 7. Insufficient horizontal and vertical communication within the government. Organizations of police administration, social administration, population and household administration, health management and environment conservation were not able to cooperate with one another smoothly. Thorough implementation of epidemic prevention and control measures could not be achieved across all levels of government, and a seamless safety net for disease control could not be established to link together central, county/city and village authorities. Based on the miserable experience and lessons of fighting SARS in the past, and combining the reports and opinions of related organizations in attendance today, the following instructions are given: 1. The above mentioned deficiencies will be reviewed one by one by administrative departments to make sure the same mistakes won’t happen again. SARS fighting was our first experience. However, if we repeat the same mistakes in the future, we will not be forgiven by the public. So please perform all preparation tasks with the most - 148 - careful attitude. Premier Hsieh must strictly supervise the implementation of all the instructions given to make sure that they are properly carried out. 2. The fundamental solution to avian flu epidemic is the research/development and stockpiling of anti-virals and vaccines. Related departments and committees should draft concrete schedules to manage the work progress of building a stockpile that meets the safety storage volume. The process should be speeded up to reach a stockpile volume sufficient for 10% of the population. In addition, related plans of priority in vaccine distribution and administration should be drafted based on different stockpile levels. 3. Human-to-human avian flu is a novel type of influenza. The health authorities should proceed with conducting relevant briefings and training for medical and public health staffs to reduce mis-diagnosis and to actively strengthen the reporting and control capabilities of the frontline epidemic prevention network. 4. Epidemic prevention is regarded as a battle. Preparation and exercises in peacetime should be given priority. Each government authority should complete related epidemic prevention battle plans and SOP within a specified timeframe, and arrange drills and onsite practices according to various scenarios as soon as possible. 5. Strengthen cooperation with nearby countries and international health organizations to actively collect information on the latest epidemic situations and on epidemic prevention strategies. Strengthen border control and quarantine of people from pandemic areas, strictly block smuggling of related fowls and animals, and provide complete health management service to poultry husbandry workers and people who may be in close contact with migratory birds or who live in areas - 149 - where migratory birds rest. 6. There are similarities and differences between avian flu and SARS epidemics. We must remember the experiences and lessons from the SARS epidemic, which taught us the importance of correct knowledge and comprehensive preparedness and response strategies. However, we should not overreact. Responsible departments should make clear policy announcements to all people. 7. All valuable opinions of today’s attendees should be incorporated into the existing “Response strategies to Possible Invasion of Avian Flu” of each responsible department to provide a basis for implementation. Second High-level National Security Meeting 31 October 2005 President Chen Shui-bian received briefings given by the Department of Health and Council of Agriculture, Executive Yuan, and joined discussions with participants. The president made a nine-point conclusion following the meeting. 1. Although the highly pathogenic avian influenza runs the risk of becoming an epidemic worldwide, the Executive Yuan has worked with all related authorities in formulating pre-emptive measures regarding prevention and quarantine of the disease and helped Taiwan to remain as a non-affected area. Taiwan also ranks No. 3 following Japan and Australia in ratings made by international risk assessment companies. I hereby express my highest affirmation to the disease prevention team of the Cabinet. 2. The Chinese government has on the one hand suppressed Taiwan from - 150 - entering international health organizations, making it difficult for us to join the worldwide effort of disease prevention. On the other hand, they have a tendency of covering up this epidemic, given their concerns on economic development, social stability and the 2008 Olympic Games. The possible lack of transparency of the disease in China will put Taiwan at the risk of extreme danger. We hereby urge the international community to join efforts and monitor the spread of the bird flu in China and to ensure that they have a well-established, fast and transparent report system, so China will not become a loophole in the world disease prevention. 3. It is fortunate that the Coast Guard Administration has seized some 1,000 smuggled birds on their way to Taiwan from China on October 14. This prevented us from facing a potential invasion of the deadly disease. We never know, however, whether birds carrying virus H5N1 have already been brought into Taiwan. We all understand that combating a disease is like waging a war. And there should be no loophole in our disease prevention network. And we are concerned that China might set off the epidemic of avian flu in Asia as it boasts to have the world's largest bird and poultry population. We must be on guard and never rule out the possibility that the bird flu might have already entered into this island, and we must do everything we could to break every possible link to the disease. We should also revise immediately related laws and regulations, intensifying the punishment of bird smuggling so as to stop the crime. 4. The making and stockpiling of antiviral drugs and vaccines have become the utmost important work regarding the disease. Whether we purchase it from abroad or make it by ourselves, we must have a satisfiable amount of drugs and vaccines that meet the safety standard - 151 - of the World Health Organization (WHO). Already in our stockpile is the Tamiflu, the internationally test-proven and most effective solution to the disease. It is meanwhile our only weapon combating the epidemic, though doubts have arisen in respect to its effectiveness. It is the responsibility of the government to teach our general public and help them understand the usefulness of the drugs. On the issue of the authority of drug production, I hope that Premier Frank Hsieh would pay more attention to the matter and solve the problem as soon as possible. 5. The paranoid about the avian influenza in Italy has caused its poultry farmers to go on strikes. But we must know that it doesn't do us good either if we overestimate or underestimate the situation. And we should re-evaluate the appropriateness of the measure announced by the Ministry of Education that students are advised from now on to take their temperature. What we should do for the time being is to educate and to equip people with the correct knowledge about the disease. Also, we must keep the international community with the correct information. Whenever wrong stories about the disease were reported in the international society, we should ask that it be corrected immediately. 6. The outbreak of avian flu in Taiwan will become not only a disaster for national health and for Taiwan's birds and poultry business, but a great threat to social stability and national security. Internationally, we should fight for the right to join efforts with the world in disease prevention. Domestically, everyone from central to local governments should work together to prevent the disease, gather useful resources, maintain enough manpower, engage in inspections and quarantines of the disease, combat bird smuggling, monitor route of migratory birds, - 152 - keep good management of domestic animals, and increase the ability to manage crises. 7. Up to 18 countries have found cases of the highly pathogenic avian influenza, with China, Vietnam and Thailand being listed as high-risk areas by the WHO. Our government should take on the responsibility and teach our nationals who plan to travel to these countries on how to save themselves from contracting the epidemic. And we also urge travelers arriving from the above-mentioned nations to exercise health self-management. 8. The strike of SARS two years ago wrecked havoc in the stock market as well as our economy. Western economists have agreed that the impact of avian flu on world economy could run beyond imagination if not handled with care. We therefore ask Vice Premier Wu Rong-yi and Minister without Portfolio Lee Ying-yuan to complete the risk assessment of countermeasures and help the government deal with emergencies. 9. Related authorities please incorporate opinions and suggestions by all participants regarding the issue of avian flu, making them as references for the government's policy-making. Third High-level National Security Meeting 9 March 2006 President Chen directed the adoptation of the following 10 points: 1. China has become a black hole of the global avian flu prevention network. Taiwan should watch more closely on China's avian flu updates, strengthen surveillance, and, more importantly, ask the international community to urge Chine to keep China's disease status - 153 - and information transparent before it's too late to control the outbreaks. 2. Highly pathogenic avian flu H5N1 is transmittable from migrant birds to domestic birds and vice versa. As Taiwan is located amid the migration routes, it should continue to keep track on this matter. Also, avian flu prevention policies should be persistent and regularized. 3. According to other countries' experiences, some important strategies against avian flu pandemics include: effective isolation of wild birds from domestic ones; culling H5N1-infected birds as soon as there is an outbreak; and preventive vaccination. If any outbreak of this sort happens in Taiwan, the central government and the local governments must work in synergy, all the orders must be fully carried out, and no negligence and procrastination are allowed to happen in any link of the chain of command. 4. We should be more stringent on inbound and outbound traffic quarantine, particularly in preventing illegal bird imports from China, blocking China's fishing boats from entering our territory, cracking down on human trafficking, and strengthening the quarantine work in the "Mini Three Links" in Kinmen and Matsu. 5. Storage and development of antivirus drugs and vaccination are most effective to fight against avian flu pandemics. Our antivirus drug storage must reach the required safety level, and the research and development capacity of vaccines must be built up in accordance with Dr. Lee Yuan-tseh's initiation proposed in 2004 APEC Leaders' Meeting. 6. Traditional markets are the blind spots in avian flu prevention. The government should work on the possibility of prohibiting butchering - 154 - poultry in traditional markets and follow the stipulations according to the Law of Animal Husbandry and thus conduct centralized butchering with machinery. 7. After examining the disease spreads in Hong Kong, Guangzhou, Hanoi, and Ho Chi Minh City, the Urbani Foundation has brought back useful information on avian flu prevention. The government should guide and assist the foundation to communicate with China in regard to disease prevention and control. 8. The key to successful disease prevention and control lies in setting correct strategies and transforming these strategies into standardized operation procedures according to which practices and examinations can be applied. Educating the public is another key area that should not be overlooked. According to the conference conclusions, the Executive Yuan is held responsible to arrange an on-the-site operation and schedule for the president to inspect. 9. In order to establish a reference tank, the Executive Yuan should, according to the conference conclusions, pre-assess any possible impact resulted from avian flu outbreaks on national defense, economy, social stability, cross-strait exchange, transnational marriage, introduction of foreign labors, etc. 10. All the related ministries and departments should adapt and adhere to the opinions presented in the conference and its conclusions, and thus incorporate the above mentioned measures into their respective counterpart strategies. - 155 - Appendix 4. Enforcement Regulations Governing the Central Epidemics Command Center Formulated and announced in 37 articles by the Department of Health, the Executive Yuan, on December 20, 2004, under Shu-Shou-Chi order No. 0930001221 Chapter 1 General Principles Article 1 This set of Regulations is formulated in accordance with regulations of Paragraph 2, Article 17 of the Communicable Disease Control Act (hereafter referred to as the Act). Article 2 If the central competent authority, in judging the severity of the epidemic situations in the country in accordance with regulations of Paragraph 1, Article 17 of the Act, decides that there are needs to consolidate resources, facilities, and to integrate personnel of organizations (institutions) concerned, may make concrete recommendations on mobilization for disease control, and reports to the Executive Yuan for approval for the establishment of a central epidemics command center (hereafter referred to as the Center), and appoints a commanding officer to meet the epidemic situations. The severity of epidemic situations mentioned in the preceding Paragraph refers to the epidemics of major communicable diseases, attacks of biological pathogenic agents, or conditions judged by the central competent authority requiring mobilization to meet emergencies. Article 3 The functions of the Center are as follows: 1. To evaluate the information of disease surveillance, to formulate and promote emergency policies for disease control; - 156 - 2. To consolidate and integrate resources, facilities, and personnel of organizations (institutions) concerned needed for meeting emergencies of disease control; 3. To conduct matters concerning news releases, information and education, use of mass media with priority, control of entry and exit of country (border), house quarantine, liaison and cooperation with international organizations, control of airports and harbors, requisition of transportation means, cleaning and disinfection of public environment, labor security and hygiene, control of communicable diseases common to humans and animals, and other necessary control measures against major communicable diseases. Article 4 The commanding officer of the Center has complete authority over, supervises, and coordinates government organizations at various levels, public enterprises, reserved servicemen’s organizations, nongovernmental organizations, to implement disease control matters; when necessary, support of the army may be coordinated. The commanding officer may assign one to three deputy commanding officers to assist in executing the functions of the Center. Article 5 The commanding officer may, in accordance with regulations of Article 50 through Article 54 of the Act, instruct government organizations at various levels to requisition, appropriate, and integrate resources, facilities or manpower of organizations (institutions) concerned. Chapter 2 Organization and Assistance - 157 - Article 6 The Center may establish a secretariat, department of execution, department of planning, department of logistics, and department of finance; each department may establish several task force sections. In each department mentioned in the preceding Paragraph, there shall be one director appointed by the commanding officer. The commanding officer may, upon needs for meeting the emergencies of disease control, flexibly adjust the task force sections of each department, their size of staff, and their timing of establishment. Organizations concerned may be requested to assist, when necessary, the functions of each department. Article 7 The Department of Health, the Executive Yuan, shall be responsible for the Secretariat. The functions are as follows: 1. Overall control of the emergency operations and management of situations, coordination, and consolidation of resources; 2. Conduct of training and exercises on emergency operations for personnel to be involved; 3. Follow-up and assessment; 4. Other matters concerning overall control. Article 8 The Executive Department may establish the following sections: 1. Disease investigation section; 2. Medical affairs section; 3. Immigration control section; 4. Foreign affairs section; 5. Information section; - 158 - 6. Control section; 7. Service section; 8. Emergency management section. Article 9 The Department of Health, the Executive Yuan, shall be responsible for the Disease Investigation Section of the Executive Department. The functions and division of support are as follows: 1. Epidemiological investigations and surveillance of community health, patients, sources of infection, or vectors; 2. Surveillance of animals, to be supported by the Council of Agriculture, the Executive Yuan; 3. Surveillance of environment, to be supported by the Environmental Protection Administration, the Executive Yuan; 4. Investigation and control of crimes, to be supported by the Ministry of the Interior; 5. Information on bio-terrorism attack, to be supported by the National Security Council and Ministry of Justice; 6. Other matters concerning disease investigations and surveillances. Article 10 The Department of Health, the Executive Yuan, shall be responsible for the Medical Affairs Section of the Executive Department. The functions and division of support are as follows: 1. Emergency care before arrival to hospital, resource control on scene, and when necessary, military medical support and care, to be supported by the Ministry of the Interior and Ministry of - 159 - National Defense; 2. Medical care, follow-up care, evacuation, liaison, reporting, to be supported by the Ministry of the Interior, Ministry of National Defense, and Ministry of Education; 3. Measures concerning medical safety and infection control; 4. Other matters concerning medical care and infection control. Article 11 The Ministry of the Interior shall be responsible for the Immigration Control Section of the Executive Department. The functions and division of support are as follows: 1. Control of entry and exit of the country (border), to be supported by the Mainland Affairs Council, the Executive Yuan; 2. Quarantine at borders, to be supported by the Department of Health, the Executive Yuan; 3. Disease control, to be supported by the Department of Health, the Executive Yuan, Mainland Affairs Council, the Executive Yuan, and Coast Guard Administration, the Executive Yuan; 4. Establishment of registration and reporting systems for passengers entering or exiting the country (border); 5. Other matters concerning immigration control. Article 12 The Ministry of Foreign Affairs shall be responsible for the Foreign Affairs Section of the Executive Department. The functions are as follows: 1. International cooperation in disease control, relief, liaison, and provision of information; - 160 - 2. Assistance to and management of aliens at risk; 3. Visa control of aliens; 4. Other matters concerning international cooperation in disease control and liaison. Article 13 The Department of Health, the Executive Yuan, and the Government Information Office, the Executive Yuan, shall be jointly responsible for the Information Section of the Executive Department. The functions are as follows: 1. Release of news concerning disease control; 2. Report of international and domestic news on disease control, and coordination of management; 3. Negotiation on the use on priority basis of mass media and communications facilities; 4. Consolidation and dissemination of information for disease control education; 5. Other matters concerning disease control information and education. Article 14 The Department of Health, the Executive Yuan, shall be responsible for the Control Section of the Executive Department. The functions and division of support are as follows: 1. Public health control measures such as immunization, house quarantine, and self health management, to be supported by the Ministry of the Interior; 2. Vector surveys and control; 3. Control and disinfection of environment, safety of water - 161 - supply, elimination of vectors and sources of breeding, to be supported by the Environmental Protection Administration, the Executive Yuan; 4. Quarantine and control of host animals of communicable diseases common to humans and animals, to be supported by the Council of Agriculture, the Executive Yuan; 5. Other matters concerning disease control. Article 15 The Department of Health, the Executive Yuan, shall be responsible for the Service Section of the Executive Department. The functions and division of support are as follows: 1. Mental health services to the public, patients, and families; 2. Handling, examination of remains which have died of communicable diseases, professional counseling or technical support, to be supported by the Ministry of the Interior and Ministry of Justice; 3. Matters concerning condolences to families of victims of communicable diseases, to be supported by the Ministry of the Interior; 4. Other matters concerning services to peatients. Article 16 The Ministry of the Interior shall be responsible for the Emergency Management Section of the Executive Department. The functions are as follows: 1. Matters concerning emergency management such as fire control of the scene of incident, search and rescue, control of order, and management of hazardous articles; 2. Maintenance of public works; - 162 - 3. Other matters concerning emergency management. Article 17 The Department of Planning may establish Information Section and Evaluation Section, to be under the responsibility of the Department of Health, the Executive Yuan. The functions are as follows: 1. Surveillance of disease epidemics and collection, compilation and analysis of real-time information for the advance warning systems; 2. Designing of information systems, negotiation, maintenance, and management of information exchanges; 3. Amendment of emergency measures and standard operational procedures and evaluation of achievements; 4. Epidemiological investigations, and formulation of emergency management, restoration, or mid-, long-term, alternative plans, and evaluation of their achievements; 5. Support to the briefing of epidemic situations and incidents; 6. Other matters concerning data processing, program planning, and evaluation of achievements. Article 18 The Department of Logistics may establish the following sections: 1. Technical section; 2. Transportation section; 3. Supply section; 4. Manpower section; 5. National defense resource section. - 163 - Article 19 The Department of Health, the Executive Yuan, shall be responsible for the Technical Section of the Department of Logistics. The functions are as follows: 1. Quality control of laboratory testing and technical support; 2. Control of communications, and maintenance of the soft and hardware of information and visual-information systems, and technical support; 3. Assessment of techniques for disaster management, maintenance of special facilities; 4. Other matters concerning disease control technology. Article 20 The Ministry of Communications shall be responsible for the Transportation Section of the Department of Logistics. The functions are as follows: 1. Requisition of sea, land, air transportation means and support to their communications technology; 2. Management of the transportation of manpower, disease control supplies and facilities; 3. Emergency transportation of specimens; 4. Other matters concerning transportation. Article 21 The Department of Health, the Executive Yuan, shall be responsible for the Supply Section of the Department of Logistics. The functions and division of support are as follows: 1. Sufficient supply and storage control of materials for disease control and medical care, to be supported by the Ministry of Economic Affairs; - 164 - 2. Supervision and control of the fair trade of materials for disease control and medical care, to be supported by the Fair Trade Commission, the Executive Yuan; 3. Appropriation of special materials and facilities for disease control and medical care, to be supported by the Ministry of Economic Affairs; 4. Other matters concerning the control of materials for disease control. Article 22 The Department of Health, the Executive Yuan, shall be responsible for the Manpower Section of the Department of Logistics. The functions are as follows: 1. Requisition and training of medical manpower; 2. Life support, medical care of employees, and assistance to their families; 3. Other matters concerning manpower support. Article 23 The Ministry of National Defense shall be responsible for the National Defense Resource Section of the Department of Logistics. The functions are as follows: 1. Provision of information on relevant hazards collected by the military information systems; 2. Monitoring and control of disease epidemics in army, and their reporting; 3. Support of isolation sites, devices, or manpower for disease control and medical care; 4. Other matters concerning support needed for disease control. - 165 - Article 24 The Department of Finance may establish the following sections: 1. Administration section; 2. Industry section; 3. Relief section; 4. Reconstruction section. Article 25 The Department of Health, the Executive Yuan, shall be responsible for the Administration Section of the Department of Finance. The functions are as follows: 1. Administrative support to biddings for procurement, financial reports, and management of the registration of properties; 2. Review, approval, and supervision of monetary awards for personnel, compensations and their procedures; 3. Security control of the Center, and installation and maintenance of business machines; 4. Other matters concerning administrative support. Article 26 The Council for Economic Planning and Development, the Executive Yuan, shall be responsible for the Industry Section of the Department of Finance. The functions are as follows: 1. Stable development of domestic economics and finance; 2. Assistance to industries to meet impacts of disease epidemics; 3. Supervision of stable commodity prices and fair trade; 4. Other matters concerning stability of industries. Article 27 The Ministry of the Interior and the Ministry of Finance shall be jointly responsible for the Relief Section of the Department - 166 - of Finance. The functions are as follows: 1. Education on policies of post-disaster reconstruction and restoration, and assessment of the costs; 2. Assistance to the relief of industries under impact; 3. Approval of documents for relief and sympathy money; 4. Abatement, exemption, or delayed payment of taxes, assistance to matters such as the settlement of disaster insurance claims or low-interest loans, to be supported by the Finance Supervision and Management Commission, the Executive Yuan; 5. Other matters concerning relief. Article 28 The Department of Health, the Executive Yuan, shall be responsible for the Reconstruction Section of the Department of Finance. When necessary, support of relevant organizations may be requested. The functions are as follows: 1. Attribution of responsibilities and explanation of public health laws and regulations; 2. Risk-management of medical care (medical) institutions, and coordination; 3. Managements of use licenses for pharmaceuticals, medical devices, and special facilities; 4. Reconstruction after disaster of medical care (medical) institutions, management of restoration, and compensations to losses of emergency appropriation; 5. Other matters concerning the reconstruction and restoration of medical care (medical) institutions. - 167 - Chapter 3 Training Article 29 As needed for meeting emergencies of disease situations, the Center may, in coordination with the emergency plans of the central competent authority, conduct special lectures and training for relevant personnel. Chapter 4 Operational Procedures Article 30 The commanding officer may, pending upon the epidemic situations, notify ministries and departments concerned to dispatch personnel to the Center by the designated time. Each organization or department concerned shall arrange a duty shift schedule for personnel to be assigned, and prepare a name list for emergency contact, and send them together to the Center. Article 31 The departments and sections of the Center shall at all times be kept aware of all new information of the situations; and upon receipt of the information, shall process it in the following ways: 1. The processing of information and recommendations for management shall be reported to the Secretariat, and notify departments concerned. 2. When cross-departmental coordination or reporting is necessary, the relevant department with authority and responsibility shall be informed immediately for prompt action; and shall, at the same time, report to the Secretariat. 3. Reporting of information with attributable responsibility, - 168 - recommendations for management, orders, supervision, and telephone calls, shall be recorded in writing, and submitted to senior officers with authority and responsibility. 4. A daily record shall be kept, and submitted to the senior officers with authority and responsibility. Article 32 The Center may send official communication to other organizations under the name of either the Center or the commanding officer. Article 33 The commanding officer may, depending upon the epidemic situations and their management, request the Executive Yuan to dissolve the Center. Article 34 Upon dissolution of the Center, the organizations which have joined the Center shall submit all records made during the existence of the Center to the central competent authority for collection and reporting; the various restoration and reconstruction measures shall be continued by the organization with authority and responsibility. Chapter 5 Supplementary Provisions Article 35 The administrative expenses of the Center shall be paid from the budget prepared by the central competent authority. Article 36 When local competent authorities establish, in order to meet epidemic situations, local epidemics commanding centers, the relevant regulations of this set of Regulations may also apply. Article 37 This set of Regulations shall be implemented on the day of announcement. - 169 - Appendix 5. Exercises in Response to Influenza Pandemic Central Government (July 2005~October 2006): 1. 7 July 2005: Functional exercise of novel influenza for pandemic phase A1~A2 (held by CDC) 2. 19 October 2005: 2005 Joint epidemic prevention exercise of highly pathogenic influenza (held by Council of Agriculture) 3. 27 December 2005: Functional exercise of novel influenza for pandemic phase B~C (held by CDC) 4. 21-22 March 2006: 2006 national defense No. 29 WanAn exercise – Suspected novel influenza (human avian flu) induced “Joint exercise of administration and army” (held by Command Department of National Reserve Force in southern area) 5. 6 April 2006: Drill on control measures for incoming passangers in the airport (held by Ministry of Transportation and Communication, Ministry of the Interior and DOH) 6. 20 April 2006: 2006 national defense No. 29 WanAn exercise – Exercise of large care facilities in novel influenza pandemic phase C (held by Ministry of National Defense and Pingtung county government) 7. 11 August 2006: Drill of anti-virals prescription (held by Bureau of Pharmaceutical Affairs of DOH) 8. 17 August 2006: Egret No.1 – Off-shore infectious disease patients exercise without prior notice – Exercise of Taipei area Infectious Disease Prevention and Control Medical Network on handling infectious disease patients in off-shore islands – “Patient doesn’t move, medical group stationing” (held by CDC) 9. 22 August 2006: Egret No. 7 – Operation of Central Epidemic - 170 - Command Center in pandemic phase A1 (held by CDC) 10. 14 October 2006: Egret NO.2 – Exercise on emergency dispatch of epidemic prevention materials (held by CDC) 11. 19 October 2006: Egret No.1 (2nd exercise) – Off-shore infectious disease patients exercise without prior notice – Exercise of Infectious Disease Prevention and Control Medical Network on handling infectious disease patients in off-shore islands – “Patient doesn’t move, medical group deployed” (held by CDC) Local Government (January~October 2006): 1. 16 January 2006: Exercise on procedures of hospital evacuation during novel influenza pandemic (held by Changhua county) 2. 8 January 2006: “Exercies on handling procedures and transfer of novel influenza patients” without prior notice (held by Hualien county) 3. 21 February 2006: 2006 Exercise on retail market and street vendor aggregated areas in response to avian flu prevention in Tainan (held by Tainan city) 4. 24 March 2006: Exercise on transportation and treatment of novel influenza cases imported through mini-three links with China (held by Kinmen General Hospital of DOH) 5. 31 March 2006: Table-top exercise of Incident Command System (ICS): Dealing with novel influenza (held by Taipei city) 6. 9 June 2006: Exercise of large care facilities in Changhua county during influenza pandemic (held by Changhua county) 7. 26 July 2006: Table-top exercise on epidemic prevention of novel influenza (avian flu) in phase A and C (held by Taipei city) 8. 26 July 2006: 2006 Exercise of large care facility mobilization during - 171 - influenza pandemic (held by Miaoli county) 9. 27 July 2006: 2006 Exercise of large care facility in influenza pandemic phase B and C (held by Hualien county) 10. 31 July 2006: 2006 Functional exercise on epidemic prevention of highly pathogenic avian flu (held by Hualien county) 11. 13 August 2006: Receiving, treatment and transfer of novel influenza patients from primary clinics (held by Taipei county Bureau of Health) 12. 27 October 2006: Exercise of pandemic large care facilities in Tainan county during pandemic (held by Tainan county) 13. 4 October 2006: Exercise of establishment of large care facilities in response to pandemic in Yilan county (held by Yilan) 14. September~October 2006: Table-top exercise of evacuation plan of Infectious Disease Prevention and Control Hospital. Twenty-three exercises in total (held by county/city governments) 15. September~October 2006: Table-top exercise on evacuation of non-infectious disease prevention and control hospitals enlisted for help by government in response to novel influenza pandemic. Six exercises in total (held by county/city governments) - 172 - Reference 1. Normile D. Evidence points to migratory birds in H5N1 spread. Science 311: 1225, 2006. 2. Enserink M. H5N1 moves into Africa, European Union, deepening global crisis. Science 311, 932, 2006. 3. Butler D and Ruttimann J. Avian influenza and the new world. Nature 441:137-9, 2006. 4. WHO Geneva:Weekly epidemiological record No.26,2006,81,249-260 5. Foreign Affairs: Preparing for the Next Pandemic, Jul-Aug 2005. http://www.foreignaffairs.org/20050701faessay84402-p0/michael-t-ost erholm/preparing-for-the-next-pandemic.html 6. APEC Ministerial Meeting on Avian and Influenza Pandemics Da Nang, Viet Nam, APEC Pandemic Response Exercise, May 2006. 7. WHO. Consultation on priority public health interventions before and during an influenza pandemic, Mar 2004. 8. WHO. WHO global influenza preparedness plan The role of WHO and recommendations for national measures before and during pandemics, May 2005. 9. WHO.Avian influenza: assessing the pandemic threat, Feb 2005. http://www.who.int/csr/disease/influenza/en/H5N1-pass.pdf 10.The United States Department of Health & Human Services. HHS Awards Contracts Totaling More Than $1 Billion To Develop Cell-Based Influenza Vaccine. Thursday, May 4, 2006. http://www.hhs.gov/news/press/2006pres/20060504.html - 173 - 11.WHO Writing Group. Nonpharmaceutical International Measures, Jan 2006. 12.US Homeland Security Council. National Strategy for Pandemic Influenza (Implementation Plan), May 2006. 13.WHO. Avian influenza, including influenza A (H5N1), in humans: WHO interim infection control guidelines for health-care facilities ii, Apr 2006. 14.WHO. WHO pandemic influenza draft protocol for rapid response and containment, Jan 2006. 15.WHO. International Food Safety Authorities Network (INFOSAN), 4 Nov 2005. 16.FAO/OIE/WHO Consultation on Avian Influenza and Human Health: Risk Reduction Measures in Producing, Marketing and Living with Animals in Asia. Kuala Lumpur, Malaysia, 4-6 Jul 2005. 17.Institute of Medicine of the National Academies, USA. Reusability of facemasks during an influenza pandemic: Facing the flu http://www.nap.edu/catalog/11637.html 18.Health Research Institute of Labor Safety: Q & A of choosing technique of dust proof masks used by high risk personnel to prevent pathogen. http://www.iosh.gov.tw/netbook/avian/avian940822.htm 19.US Center for disease control. Business pandemic influenza planning checklist, Dec 2005. 20.WHO. Exercise Development Guide for Validating Influenza Pandemic Preparedness Plans, Feb 2006. 21.website: http://www.fema.gov/. - 174 - 22.Chang Yu-Ning. “Exercise symposium – Take flu pandemic as example” teaching materials. CDC, National Defense University Department of Army. 23.WHO. H5N1 avian influenza: timeline, May 2006. 24.WHO. Influenza pandemic plan. The role of WHO and guidelines for national and regional planning, Apr 1999. 25.Hien TT et al. Avian influenza A (H5N1) in 10 patients in Vietnam. N Engl J Med 350:1179–88, 2004. 26.Li KS et al. Genesis of a highly pathogenic and potentially pandemic influenza virus in eastern Asia. Nature 430: 209–13, 2004. 27.Chen H et al. The evolution of H5N1 influenza viruses in ducks in southern China. Proc Natl Acad Sci USA 101: 10452–7, 2004. http://www.sciencemag.org/cgi/content/abstract/1102287 28.Apisarnthanarak A et al. Atypical avian influenza (H5N1). Emerg Infect Dis 10:1321–4, 2004. 29.WHO. Assessment of risk to human health associated with outbreaks of highly pathogenic H5N1 avian influenza in poultry, May 2004. 30.Verbal report at WHO international consultation. 31.WHO. Vaccines for pandemic influenza: informal meeting of WHO, influenza vaccine manufacturers, national licensing agencies, and government representatives on influenza pandemic vaccines, Nov 2004. http://www.who.int/csr/resources/publications/influenza/WHO_CDS_ CSR_GIP_2004_3/en/ 32.Kuiken T et al. Avian H5N1 influenza in cats. http://www.sciencemag.org/cgi/content/abstract/1102287 - 175 - 33.Issued (prior to publication) on WHO website: Avian influenza – situation in Asia: altered role of domestic ducks, 29 Oct 2004. http://www.who.int/csr/don/2004_10_29/en/index.html 34.WHO. WHO Influenza Pandemic Preparedness Checklist, Nov 2004. 35.Ungchusak K et al. Probable person-to-person transmission of avian influenza A (H5N1). N Engl J Med 352:4333–40, 2005. 36.WHO. Influenza pandemic preparedness and response, 20 Jan 2005. 37.De Jong M et al. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N Engl J Med 352:7686–91, 2005. 38.Liu J et al. Highly pathogenic H5N1 influenza virus infection in migratory birds. ScienceExpress, 2005. http://www.sciencemag.org/cgi/contents/abstract/1115273 39.Chen H et al. H5N1 virus outbreak in migratory waterfowl. Nature 436: 191–2, 2005. 40.Taubenberger JK et al. Characterization of the 1918 influenza virus polymerase genes. Nature 437: 889–93, 2005. 41.Tumpey TM et al. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science 310: 77–80, 2005. 42.Chen H et al. Establishment of multiple sublineages of H5N1 influenza virus in Asia: 25. implications for pandemic control. Proc Natl Acad Sci USA 103: 2845–50, 2006. 43.Shinya K et al. Influenza virus receptors in the human airway. Nature 440: 435–6, 2006. 44.1van Riel D et al. H5N1 virus attachment to lower respiratory tract. Science Express 23 Mar 2006. http://www.sciencemag.org/cgi/content/abstract/1125548 - 176 - 45.Menno D. de Jog, Tran Tinh Hien. Avian influenza A (H5N1). Journal of Clinical Virology 35:2-13, 2006. 46.Shih Shui et al. “Excerpt report on suggestion to infection control of medical facilities in response to avian flu”. Epidemic Bulletin 22 (7):488-493, 2006 。 http://www.cdc.gov.tw/internet-cdc/Epidemic Bulletin/files/Vol.22/Chinese edition 22-07.pdf 47.WHO. Advice on use of Oseltamivir. WHO, 2006. http://www.who.int/csr/disease/avian_influenza/guidelines/useofoselta mivir2006_03_17A.pdf - 177 -