* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Hist PeriodicTable 2014
Alkali metal wikipedia , lookup
Dmitri Mendeleev wikipedia , lookup
Group 12 element wikipedia , lookup
Boron group wikipedia , lookup
Group 3 element wikipedia , lookup
Alkaline earth metal wikipedia , lookup
Period 3 element wikipedia , lookup
Period 6 element wikipedia , lookup
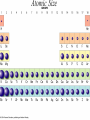
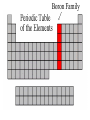
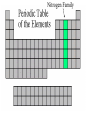
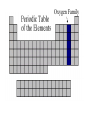
The History of the Modern Periodic Table During the nineteenth century, chemists began to categorize the elements according to similarities in their physical and chemical properties. This is what led to out modern Periodic Table Johann Wolfgang Döbereiner 1829 • Grouped known elements into families of triads. • The elements in a triad had similar chemical properties and orderly physical properties. • Li Ca S Cl Mn Na Sr Se Br Cr K Ba Te I Fe John Newlands In 1863, he suggested that elements be arranged in “octaves” because he noticed (after arranging the elements in order of increasing atomic mass) that certain properties repeated every 8th element. 1838 - 1898 Law of Octaves Dmitri Mendeleev In 1869 he published a table of the elements organized by increasing atomic mass. Considered the Father of the Periodic Table. 1834 - 1907 • Grouped elements on the basis of similar chemical properties. • Left blank spaces to add new elements where he predicted they would occur. Accepted minor inversions when placing the elements. Predicted properties for undiscovered elements, allowing for his theories to be tested. Mendeleev’s Periodic Table Henry Moseley In 1913, through his work with X-rays, he determined the actual nuclear charge (atomic number) of the elements. He rearranged the elements in order of increasing atomic number. 1887 - 1915 After co-discovering 10 new elements, in 1944 he moved 14 elements out of the main body of the periodic table to their current location below the Lanthanide series. These became known as the Actinide series. Glenn T. Seaborg 1912 - 1999 Why We Use the Periodic Table: • Identify elemental characteristics • Classify all matter • Predict reactions between elements Elemental Characteristics The Periodic Table shows: • Protons, neutrons, and electrons • Class order and valence shell electrons • Average mass of elements (mass number) Periodic Table Geography Periodic Law When elements are arranged in order of increasing atomic number, there is a periodic pattern in their physical and chemical properties. NONMETALS METALLIODS METALS Metals, Nonmetals, & Metalloids Periodic tables have a “stair step line” which allows you to identify which elements are metals, nonmetals and metalloids. Metals • Most elements are metals. • Elements to the left of the stair step line are metals or metal like elements. Physical Properties of Metals: • Luster (shininess) • Good conductors of heat and electricity • High density (heavy for their size) Physical Properties of Metals: • High melting point • Ductile (most metals can be stretched into thin wires) • Malleable (most metals can be hammered into thin sheets) Chemical Properties of Metals: • Easily lose electrons • Corrode easily. Corrosion is a gradual chemical reaction that wears away metal. (Example: silver tarnishing and iron rusting) Nonmetals • Nonmetals are found to the right of the stair step line. Their characteristics are opposite of metals. Physical Properties of Nonmetals: • • • • • • • No luster (dull appearance) Poor conductor of heat and electricity Brittle (breaks easily) Not ductile Not malleable Low density Low melting point Chemical Properties of Nonmetals: • Tend to gain electrons. • Since metals usually lose electrons and nonmetals tend to gain electrons, they like to form compounds with each other. • These compounds are called ionic compounds. • When two or more nonmetals bond with each other, they form a covalent compound. Metalloids • Elements on both sides of the “stair step line” have properties of both metals and nonmetals. These elements are called metalloids. Physical Properties of Metalloids: • • • • • Solids Can be shiny or dull Ductile Malleable Conduct heat and electricity better than nonmetals but not as well as metals Periods and Groups • Sizes of the atoms decrease as we move from left to right across a period. • This is due to the increasing number of protons in the nucleus, so the electrical attraction between the nucleus and the orbiting electrons gets stronger and pulls the electrons closer to the nucleus Atomic Size Periods The horizontal rows of the periodic table are called PERIODS. Periods • The elements in a period are not alike in properties. • The first element in a period is always an extremely active solid. The last element in a period, is always an inactive gas. • Are arranged horizontally across the periodic table (rows 1-7) Periods Continued • The period number of an element signifies the highest energy level an electron of that element occupies. • The elements in the same period have the same number of valence shells. (outermost path of an electron) The elements in any group of the periodic table have similar physical and chemical properties. The vertical columns of the periodic table are called GROUPS or FAMILIES. Families on the Periodic Table • Families may be one column, or several columns put together. • Families have names rather than numbers. (Just like your family has a common last name.) Hydrogen Hydrogen • Hydrogen is in its own family. • A diatomic, reactive gas. • Simplest and lightest of all elements. Hydrogen • Extremely flammable. It is what the sun uses to create light and heat. • Hydrogen is promising as an alternative fuel source for automobiles Alkali Metals Alkali Metals • 1st column on the periodic table (Group 1) not including hydrogen. • Most reactive metals. • Soft enough to cut with a butter knife Alkali Metals Continued • They react violently with water. • Alkali metals are never found as free elements in nature. They are always bonded with another element. Alkaline Earth Metals Alkaline Earth Metals • Second column on the periodic table. (Group 2) • Reactive metals that are always combined with nonmetals in nature. Alkaline Earth Metals • They have two valence electrons. • Several of these elements are important nutrients for people (such as Mg and Ca) What does it mean to be reactive? • Reactive elements bond easily with other elements to form compounds. • Some elements are only found in nature bonded with other elements. What makes an element reactive? • An incomplete valence electron level. • The “Octet Rule” states that atoms will gain, lose or share electrons to have the same number of electrons as their nearest noble gas. • Exceptions: Hydrogen, Beryllium, Boron What makes an element reactive? • Atoms bond until this level is complete. • Atoms with few valence electrons, lose during bonding. • Atoms with 6 or 7 valence electrons bond to gain electrons. Transition Metals Transition Metals • The compounds of transition metals are usually brightly colored and are often used to color paints. • Have 1 or 2 valence electrons, which they lose when they form bonds. Transition Metals • They have similar properties to each other and other metals, but their properties do not fit any other family. • Many combine chemically with oxygen to form compounds called oxides. Transition Metals • These are the metals you are probably most familiar with: copper, tin, zinc, iron, nickel, gold, and silver. • They are good conductors of heat and electricity. These elements are also called the Inner Transition Metals. Rare Earth Elements Rare Earth Elements • These thirty elements make the lanthanide and actinide series. • Lanthanides are naturally occurring heavy metals, used to date rocks from outer space and used in lasers. Rare Earth Elements • All Actinides are dangerously radioactive. • Only two occur in nature – the rest are made in nuclear reactors and particle accelerators. They decay or break down very quickly. Boron Family Boron Family • The Boron Family is named after the first element in the family. • Atoms in this family have 3 valence electrons. Boron Family • This is the only metalloid in the family, the rest are metals. • This family includes the most abundant metal in the earth’s crust (aluminum). Carbon Family Carbon Family • Atoms of this family have 4 valence electrons. • This family includes a non-metal, carbon, metalloids, and metals. Carbon Family • The element carbon is called the “basis of life.” There is an entire branch of chemistry devoted to carbon compounds called organic chemistry. Nitrogen Family Nitrogen Family • The nitrogen family is named after the element that makes up 78% of our atmosphere. • This family includes non-metals, metalloids, and metals. Nitrogen Family • Atoms in the nitrogen family have 5 valence electrons. They tend to share electrons when they bond. • Other elements in this family are phosphorus, arsenic, antimony, and bismuth. Oxygen Family Oxygen Family • Atoms of this family have 6 valence electrons. • Most elements in this family share electrons when bonding. • Oxygen is the most abundant element in the earth’s crust. It is extremely reactive and combines with almost all elements. Halogens Halogen Family • The elements in this family are fluorine, chlorine, bromine, iodine, and astatine. • Halogens have 7 valence electrons, this is why they are the most reactive non-metals. They are never found free in nature. • They react with alkali metals to form salts. Halogen Family • Halogen atoms only need to gain 1 electron to fill their outermost energy level. Noble Gases The Noble Gases • Noble Gases are colorless gases that are extremely un-reactive. • They are inactive because their outermost energy level is full. • Noble gasses are called “inert” because they do not easily combine with other elements. • Noble gases include: helium, neon, argon, krypton, xenon and radon. • All the noble gases are found in small amounts in the earth's atmosphere.