* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 21 Transition Metals and Coordination Chemistry
Bond valence method wikipedia , lookup
Hydroformylation wikipedia , lookup
Cluster chemistry wikipedia , lookup
Jahn–Teller effect wikipedia , lookup
Metal carbonyl wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Spin crossover wikipedia , lookup
Stability constants of complexes wikipedia , lookup
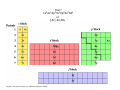
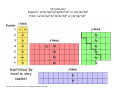
Chapter 21 Transition Metals and Coordination Chemistry Some History In the 19th century, chemists started to prepare colored compounds containing transition metals and other substances like ammonia, chloride, water and cyanide They were very interesting because their formulae gave little clue as to how they were bonded together Example: Co(NH3)6Cl3 Sophus Mads Jørgensen (1837-1914) Chain Theory Metal ammonia complexes contain chains of ammonia molecules connected to metal with chlorides at the end of each chain Alfred Werner (1866-1919) Coordination Theory Octahedral arrangement of ammonia molecules around metal forming the complex ion Co(NH3)63+ along with three Cl- ions 3Cl- Complex Ions A complex ion is a charged species consisting of a metal ion (most commonly a transition metal) surrounded by a number of molecules or ions called ligands A ligand is a Lewis base having a lone pair of electrons that can be donated to an empty orbital on the metal ion (acting as a Lewis acid) forming a coordinate covalent bond The most common ligands are H2O, NH3, Cl- and CNThe charge on a complex ion is the sum of the charges of the metal ion and the ligands Examples: CoCl42- = Co2+ + 4ClNi(NH3)62+ = Ni2+ + 6NH3 The ligands do not all have to be the same Example: Cu(NH3)4(H2O)22+ Coordination Number The number of ligands attached to the metal ion is called the coordination number The most common coordination numbers are 6, 4 and 2 Examples: Co(H2O)62+, CoCl42-, Ag(NH3)2+ Complex Ion Shapes Coordination number 6 complexes will always be octahedral Coordination number 2 complexes will always be linear Coordination number 4 complex can be tetrahedral or square planar Octahedral Complexes Tetrahedral Complexes Square Planar Complexes Linear Complexes [H3N: Ag :NH3]+ [Ag(NH3)2]+ VSEPR Theory and Complex Ions Valence Shell Electron Pair Repulsion (VSEPR) theory works for six and two coordinate complex ions but cannot be used to predict whether a four coordinate complex will be tetrahedral or square planar! Coordination Compounds A coordination compound consists of a complex ion along with one or more counterions which are anions or cations required to produce a compound with no net charge Square brackets are placed around the complex ion to indicate the composition of the complex ion Counterions are shown outside the brackets Example: [Co(NH3)5Cl]Cl2 = Co(NH3)5Cl2+ + 2ClA coordination compound behaves like any other ionic compound when dissolved in water forming independent cations and anions: [Co(NH3)5Cl]Cl2(s) Co(NH3)5Cl2+(aq) + 2Cl-(aq) H 2O Colors of Coordination Compounds Many coordination compounds are very colorful since the transition metal ions absorb light in the visible spectrum Ligand Types Ligands that can form one bond to a metal ion are called monodenate or unidentate (“single toothed”) Ligands with more than one atom with a lone pair that can bond to a metal are said to chelating ligands or chelates (“clawed”) A ligand that can form two bonds to a metal ion is called a bidentate ligand Ligands that can form more than two bonds are called polydentate Etheylenediaminetetraacetate (EDTA) A hexadenatate ligand! Common Ligands Naming Coordination Compounds Isomerism in Coordination Compounds Occurs when two or more complex ions contain exactly the same types and numbers of atoms (hence same formula) but the arrangement of ligands differ leading to different physical and/or chemical properties Linkage Isomerism The composition of the complex is the same but one or more of the ligands attaches with a different atom Example: [Co(NH3)4(NO2)Cl]Cl [Co(NH3)4(ONO)Cl]Cl Geometrical Isomerism Occurs when ligands occupy different spatial positions around the central atom cis trans trans cis Review of Electronic Configurations of Transition Metal Atoms Iron? 1s22s22p63s23p64s23d6 or [Ar] 4s23d6 Chromium? Expect? 1s22s22p63s23p64s23d4 or [Ar]4s23d4 Find: 1s22s22p63s23p64s13d5 or [Ar]4s13d5 Half-filled 3d level is very stable! Gadolinium? 1s22s22p63s23p64s23d104p65s24d105p66s25d14f7 or [Xe]6s25d14f7 Electronic Configurations of Transition Metal Ions In neutral transition elements, the s- and d-orbitals have very similar energies However, in transition metal ions the energy of the d-orbitals is significantly lower than that of the s-orbitals so that the electrons remaining after the ion forms occupy the lower energy d-orbitals. Example: Fe3+ is [Ar]3d5 not [Ar]4s23d3 Bonding in Coordination Compounds Can be considered a Lewis acid-base reaction where lone pairs on the ligands are shared with orbitals on the metal ion




































![Coordination Compounds [Compatibility Mode]](http://s1.studyres.com/store/data/000678035_1-c20c75fd4abb97d3ba4a0b0fce26e10b-150x150.png)