* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Alzheimer`s Disease and Other Dementias
Survey
Document related concepts
Transcript
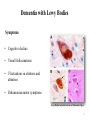
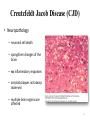
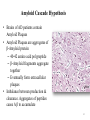
Alzheimer’s and Other Dementias PSYCH 7955 W Klugh Kennedy, PharmD, BCPP, FASHP, FCCP Clinical Professor of Pharmacy Practice and Psychiatry Mercer University, Savannah Georgia [email protected] 1 Definitions • Dementia (n): a chronic or persistent disorder of the mental processes caused by brain disease or injury and marked by memory disorders, personality changes, and impaired reasoning. • Delirium (n): an acutely disturbed state of mind that occurs in fever, intoxication, and other disorders and is characterized by restlessness, illusions, and incoherence of thought and speech. What is Dementia? • Dementia is characterized by a loss of, or decline in memory and other cognitive abilities. • It is caused by various diseases and conditions that result in damaged brain cells. – – – – Neurodegeneration Hypoxia Infection Other 3 Associated with Dementia – Parkinson's Disease – Hydrocephalus – Multiple Sclerosis – Vitamin deficiency – Alcohol – Medications – Stroke – Huntington’s Disease – Subdural hematoma – Brain tumor – Infection – Inflammation – Hormonal loss – Major organ failure 4 Types of Dementia • Alzheimer’s Disease • Dementia with Lewy Bodies • Vascular Dementia • Creutzfeldt-Jakob Disease • Reversible causes of dementia – B12 deficiency – Hypothyroidism – Depression (pseudodementia) Alzheimer’s Association http://www.Alz.org USA National Institutes on Health http://www.NIH.gov 6 Dementia with Lewy Bodies • Found primarily in individuals with Parkinson’s Disease • Lewy Bodies observable in brainstem and cortex in 25% of patients with dementia • Lewy Bodies are intracellular cytoplasmic inclusions • Composed of neurofilament proteins, ubiquitin, and α-synuclein 7 Dementia with Lewy Bodies Symptoms • Cognitive decline • Visual Hallucinations • Fluctuations in alertness and attention • Parkinsonian motor symptoms Lewy Bodies found in Neurons of Substantia Nigra 8 Vascular Dementias • Range of cognitive disorders caused by vascular disease • Most common is occlusion of cerebral blood vessels leading to brain injury • Multiple large or small strokes can lead to multi-infarct dementia (MID) 9 Creutzfeldt Jacob Disease (CJD) • Transmissible Spongiform Encephalopathies (TSEs) – Bovine Spongiform Encephalopathy (Mad Cow Disease) – Kuru • Infectious Agent – Prion • Early and late stage dementia symptoms as well as – Extrapyramidal Symptoms – Myclonus – Dizziness • No current treatment exists 10 Creutzfeldt Jacob Disease (CJD) • Neuropathology – neuronal cell death – spongiform changes of the brain – no inflammatory responses – amyloid plaques not always observed – multiple brain regions are affected 11 What is Dementia? • It must include a decline in memory (amnesia) and at least one of the following: – Aphasia: Inability to generate coherent speech or understand spoken or written language – Agnosia: Inability to recognize or identify objects, assuming intact sensory function – Apraxia: Inability to execute motor activities, assuming intact motor abilities – Decline in Executive functioning: Decline in ability to think abstractly, make sound judgments, and plan and carry out complex tasks 13 Prevalence • US: 3.5-16.1% of persons aged ≥ 65 yrs suffer from some sort of dementia • AD accounts for 50-60% of those dementias • 5.3 million in US with AD – Projected 13.2 million by 2050 • Incidence increases with age – 10% > 65 yrs • 7% of those 65-74 yrs • 53% of those 74-85 yrs 14 Prevalence of AD 15 Prevalence of AD 16 Nussbaum RL, Ellis CE. Alzheimer’s disease and Parkinson’s disease. NEJM. 2003; 348:1356–1364. Human Cost of AD • Life expectancy after onset of symptoms 3-20 yrs. – Average 8 yrs • Life expectancy decreases by 69% after diagnosis (<70 yrs) • 4th leading cause of death in US 17 Financial Cost of AD • Estimated annual direct cost: $48,000 • Estimated annual total cost: $174,000 • Estimated annual total US cost: $148 billion 18 Family Impact • 5 million caregivers of AD patients • 46% ↑ in MD visits by caregivers of dementia patients • ↑ distress levels • ½ caregivers express mild to moderate distress • 16% have high distress • ↑ levels of financial strain • Interference with employment 19 Etiology of AD – Environmental / Risk Factors • Increased Age • Decreased Reserve Capacity of Brain – Small brain size or head circumference – Low education level – Reduced mental or physical activity • Head injury – Concussion awareness • Increased risk for vascular diseases – – – – – – Hypercholesterolemia Hypertension Atherosclerosis Obesity Diabetes Smoking 20 Etiology of AD - Genetics • Early-Onset Familial Alzheimer’s Disease (FAD) – < 65 yrs – Faster progression – < 5% cases • Mutations in dominant alleles on 3 chromosomes – Chromosome 1: PSEN 2: Presenilin 2 – Chromosome 14: PSEN 1: Presenilin 1 (most aggressive) – Chromosome 21: APP: Amyloid Precursor Protein (APP) 21 Etiology of AD - Genetics • Late-Onset Alzheimer’s Disease – Apolipoprotein E (ApoE) • Cholesterol and lipoprotein metabolism – Chromosome 19 • 3 alleles – ε2 – appears to confer protection against AD – ε3 – most common – ε4 – increased risk of AD – ApoE4 • Single ε4 allele, then 47% risk of AD at 80 yrs • Both alleles ε4, then 91% risk of AD by 80 yrs • No copies of ε4 allele, then 20% risk of AD 22 Pathophysiology of Alzheimer’s Disease • Two signature pathological findings – Amyloid Plaques – Neurofibrillary Tangles (tau proteins) • Other factors – Inflammation – Reduction in Cholinergic Activity – Excitotoxicity 23 Amyloid Cascade Hypothesis • Brains of AD patients contain Amyloid Plaques • Amyloid Plaques are aggregates of β-Amyloid protein – 40-42 amino acid polypeptide – β-Amyloid fragments aggregate together – Eventually form extracellular plaques • Imbalance between production & clearance. Aggregates of peptides cause A β to accumulate 24 Amyloid Cascade Hypothesis • Normal processing of Amyloid Precursor Protein (APP) involves cleavage by α-Secretase then γ-Secretase 25 Amyloid Cascade Hypothesis • Amyloid Precursor Protein (APP) – Proteolysis by β-Secretase then γ-Secretase – Results in β-Amyloid polypeptides of length 40-42 amino acids in length – Release of β-Amyloid fragments into extracellular space – β-Amyloid fragments aggregate to form Amyloid Plaques • 42 length β-Amyloid fragment believed responsible for plaque formation – Results in toxicity and degeneration of neurons – Alzheimer’s Disease 26 Amyloid Cascade Hypothesis • Abnormal processing of Amyloid Precursor Protein (APP) involves cleavage by β-Secretase then γ-Secretase 27 Amyloid Cascade Hypothesis γ -Secretase complex γ-Secretase Complex 28 Neurofibrillary Tangles (NFTs) • Found inside neurons of AD patients – Especially in hippocampus & cerebral cortex – Found in Substantia Nigra of Parkinson’s Patients • Tau protein - provides support to microtubules • NFTs consist of hyperphosphorylated Tau protein – these cannot bind to microtubules so the microtubules collapse • Hyperphosphorylated Tau aggregates together to form NFTs which fills cytoplasm – Do these NFTs cause cell death? Or are NFTs the consequence of some other process? 29 Formation of Neurofibrillary Tangles 30 Neurofibrillary Tangles (Tau Proteins) 31 Apolipoprotein E4 and AD • Studies suggest ApoE has a role in the clearance of β-Amyloid • Enhances proteolytic breakdown of Aβ – ApoE incorporated into lipoprotein particle – ApoE then binds soluble β-Amyloid in isoform dependent manner: E2 > E3 > E4 – ApoE then endocytosed by various cells in CNS – β-Amyloid removed from brain • APOE4 not as effective as others Increased Aβ 32 Inflammation • Evidence: – Increased levels of pro-inflammatory mediators in AD (e.g. cytokines) – Epidemiological studies show NSAIDs may reduce AD risk • Hypothesis: – Inflammation occurs due to β-Amyloid accumulation – Inflammatory mediators (cytokines, NO, complement) injure neurons – Results in cell death and neurodegeneration • Clinical Trials: – NSAIDs as treatment or prevention have been disappointing 33 Cholinergic Hypothesis • Loss in cholinergic neurons reason for decline in memory and cognition and correlates with AD severity • However….. – Cholinergic cell loss a CONSEQUENCE of AD and not a cause – Other types of neurons lost in addition to cholinergic neurons • Other Neurotransmitter Abnormalities – Reduction in Serotonergic neurons of Raphe Nuclei – Reduction in Noradrenergic neurons of Locus Ceruleus 34 Excitotoxicity • Glutamate is a major excitatory neurotransmitter in CNS – Normal levels: aids in memory & learning – Increased levels: Over-stimulate nerve cells killing them through Excitotoxicity • Excitotoxicity is mediated via increased intracellular Ca2+ • Abnormalities of glutamate pathways found in cortex and limbic system in AD patients • Level of involvement in AD, if any, still unclear 35 Excitotoxicity – NMDA receptor So What? Who Cares? 36 Vascular Disease and Cholesterol • Epidemiological link between CVD and AD • Dysfunctional blood vessels: – May impair delivery of nutrients and reduce clearance of β-Amyloid – May increase β-Amyloid toxicity to neurons • Elevated cholesterol may alter membrane function and initiate Amyloid Cascade 37 Other theories • Oxidative stress and formation of free radicals – Studies suggest Vitamin E and C may help prevent AD • Mitochondrial dysfunction – Disruption of energy metabolism in neuron • Loss of estrogen in postmenopausal women – Incidence AD higher in women than men – Estrogen can directly or indirectly affect the brain • • • • Neuroprotection Neurotransmitter effects Reduce βA in brain Increase cerebral blood flow – However, clinical trials using HRT have thus far proven disappointing 38 The Big Picture • Faulty processing of APP leads to β-Amyloid – Soluble βA monomers and oligomers lead to neuronal dysfunction and cell death • Aggregation of Tau Protein leads to Neurofibrillary Tangles – Production of NFTs results in inability of cell to hold structure • Causative factor in neurodegeneration, or consequence of some other process? • Possible involvement by inflammatory mediators • Cholinergic dysfunction: a consequence of neurodegeneration • Excitotoxicity contributing factor 39 The Big Picture 40 The Big Picture 41 Diagnosis • Under diagnosed in primary care setting • Usually brought to the attention of a primary care physician by family member • Definitive diagnosis only made at autopsy • However, criteria have been developed to detect and diagnose dementia – DSM-V, AAN guidelines, etc 42 Diagnosis • Revised diagnostic and research criteria for AD • Three phases proposed: 1) Asymptomatic, preclinical AD For research purposes 2) Symptomatic, pre-dementia (MCI due to AD) 3) Dementia due to AD 43 Diagnosis • Rule out: – Other neurological conditions that cause cognitive deficits (e.g. Brain Tumor) – Other psychiatric conditions that cause cognitive deficits (e.g. MDD, Schizophrenia, delirium) – Systemic conditions that cause cognitive deficits (e.g. Hypothyroidism, anemia) – General Medical Conditions – Substance Related Disorders 44 Diagnostic Tests • CT scan – useless • MRI scan – probably useless • PET, SPECT, fMRI – Still experimental, maybe useless • Routine Lab Tests – – – – – – – CBC CMP, BMP LFTs Thyroid Vitamin B12 Syphilis – RPR, VDRL UA • Lumbar Puncture and analysis of CSF for tau and B-amyloid proteins. – probably useless, not with out risks 45 Assessment Tests • Folstein Mini-Mental State Examination? • Alzheimer’s Disease Assessment Scale – Cognitive (ADAS-Cog) • Alzheimer’s Disease Cooperative Study – Activities of Daily Living (ADCS-ADL) • Others 46 Mini Mental State Exam • Tests on 5 areas of cognition – Orientation – Registration – Attention – Recall – Language 47 Clinical Presentation of AD Cognitive Symptoms Noncognitive Symptoms • • • • • • Depression • Psychosis • Behavioral Disturbances Memory Loss Aphasia Agnosia Disorientation Impaired Executive Function 48 Signs of AD 49 Alzheimer’s Association. Symptoms of Alzheimer’s. Available at: www.alz.org/alzheimers_disease_symptoms_of_alzheimers.asp.. Disease Progression • Onset of AD generally subtle • Stages: – Mild: forgets names of places, word-finding difficulties, trouble using household appliances, loss of interest in activities – Moderate: forget recent events, has trouble handling money, agitated, starts walking or roaming about – Severe: has trouble using or understanding words, lack of self-care functions 50 Disease Progression 51 Sadik K. Wilcock G. The increasing burden of Alzheimer’s disease. Alzheimer Dis Assoc Disord. 2003;17:S75–S79. Stages of Alzheimer’s Disease 52 7 Stages of Alzheimer’s • Stage 1: No impairment (normal function) • Stage 2: Very mild cognitive decline – May be normal age-related changes or the earliest signs of AD – Memory lapses • Forgetting words, names, location of keys, etc. • Not apparent to others or evident during medical examination http://www.alz.org/alzheimers_disease_stages_of_alzheimers.asp 7 Stages of Alzheimer’s • Stage 3: Mild Cognitive Decline – Early AD can be dx in some patients – Friends / family begin to notice deficits – Problems with word or name-finding – Performance issues at work noticeable – Decline in ability to plan / organize – Lose / misplace valuable object http://www.alz.org/alzheimers_disease_stages_of_alzheimers.asp 7 Stages of Alzheimer’s • Stage 4: Moderate Cognitive Decline (Mild AD) – Medical exam detects clear-cut deficiencies: • Decreased knowledge in current / recent events • Impaired ability to perform mental arithmetic (serial 7s) • Decreased capacity to perform complex tasks (pay bills, manage finances) • Reduced memory of personal history – May seem withdrawn http://www.alz.org/alzheimers_disease_stages_of_alzheimers.asp 7 Stages of Alzheimer’s • Stage 5: Moderately Severe Cognitive Decline (Moderate AD) – Major gaps in memory and deficits in cognitive function are very evident – May be unable to recall address, telephone #, name of HS or college – Difficulty with simple mental arithmetic (ex. counting down by 2s) – Need help in selecting appropriate clothing for season – Usually know own name & name of spouse/kids – No assistance needed with eating or toileting http://www.alz.org/alzheimers_disease_stages_of_alzheimers.asp 7 Stages of Alzheimer’s • Stage 6: Severe Cognitive Decline (Moderately severe AD) – Significant personality changes emerge – Need assistance with ADLs – Lose awareness of recent experiences / events – May be unable to recall name of spouse / kids but can distinguish familiar faces – Need help getting dressed (errors such as putting shoes on wrong feet, putting PJs over regular clothes) http://www.alz.org/alzheimers_disease_stages_of_alzheimers.asp 7 Stages of Alzheimer’s • Stage 6 (Cont.) – Major changes in sleep patterns – Need assistance with toileting (wiping, flushing) – Increased episodes of urinary or fecal incontinence – Significant personality changes / behaviors • Paranoia or other delusions • Hallucinations • Repetitive behaviors (hand-wringing, vocalizations) – Wandering http://www.alz.org/alzheimers_disease_stages_of_alzheimers.asp 7 Stages of Alzheimer’s • Stage 7: Very Severe Cognitive Decline (Severe or End-Stage AD) – Final stage – Lose the ability to respond to their environment, ability to speak, ability to control movement – Lose the ability to walk without assistance, site without support, hold head up, smile. – Reflexes abnormal, muscles grow rigid – Impaired swallowing http://www.alz.org/alzheimers_disease_stages_of_alzheimers.asp Treatment of Dementia GOALS: • • • • Prevent or delay disease progression Maintain cognitive function Reduce behavioral symptoms Maintain or improve functional abilities 60 Treatment of Dementia • Nonpharmacotherapy – Education – Behavioral Interventions – Communication – Planning 61 Treatment of Dementia • Pharmacotherapy of Cognitive Symptoms – Cholinesterase Inhibitors • tacarine, donepezil, rivastagmine, galantamine – NMDA Antagonists • Memantine (namenda) – Potential or alternative treatments: • • • • Estrogens NSAIDs Lipid Lowering Agents Vitamin E 62 Drug name Brand name 1. donepezil Aricept Approved For FDA Approved All stages 1996 2. galantamine Razadyne Mild to moderate 2001 3. memantine Namenda Moderate to severe 2003 4. rivastigmine Exelon All stages 2000 5. donepezil and memantine Namzaric Moderate to severe 2014 Knowledge Check Which of the following are goals of dementia treatment? A. Reverse disease progression B. Restore cognitive function C. Eliminate behavioral symptoms D. Maintain or improve functional abilities Cholinesterase Inhibitors • Neurodegeneration leads to reduction in cholinergic activation in AD • Cholinergic neurons activated through nicotinic and muscarinic receptors in CNS • Acetylcholine release from vesicles in presynaptic neuron • Activate receptors on postsynaptic neuron • Some ACh hydrolyzed via Acetylcholinesterase • Cholinesterase Inhibitors block Acetylcholinesterase thus increasing ACh concentration in synaptic cleft • Results in increased cholinergic activation 65 Cholinesterase Inhibitors Acetylcholinesterase 66 Cholinesterase Inhibitors • Cholinesterase inhibitors bind REVERSIBLY to AchE resulting in accumulation of ACh. • FDA approved Cholinesterase Inhibitors – Tacrine (Cognex®) – Donepezil (Aricept®) – Galantamine (Razadyne®) – Rivastigmine (Exelon®) 67 Cholinesterase Inhibitors (Warnings) • Neurological effects – Exacerbate or induce extrapyramidal symptoms; worsening of PD tremor – May potentiate seizures • Peptic ulcers / GI bleeding due increased acid secretion • Anesthesia with succinylcholine type muscle relaxation – DC short-term if surgery planned • Cardiac conduction effects – Caution if h/o bradycardia / syncope • Urinary obstruction • Asthma / obstructive pulmonary disease 68 Tacrine (Cognex®) • 1st approved for AD • Readily crosses BBB • Modestly improves cognitive symptoms • Significant side effects – N&V – Elevations in LFTs • Dosing • ADME – Rapidly absorbed after oral administration – Absorption reduced by food – 17% Bioavailability – Metabolized via CYP450 – T ½ : 2-4 hours – 10 mg QID to max 160 mg/day • Not used clinically 69 Donepezil (Aricept®) • • • • • Approved for mild, moderate, & severe AD Readily crosses BBB Little effect in periphery Modestly improves cognitive symptoms AE profile • 5-10% GI upset, insomnia, N&V, diarrhea, decreased appetite, weight loss • Dosing • 5 - 10 mg mild to moderate AD • 10 - 23 mg moderate to severe AD 70 Donepezil (Aricept®) • ADME • • • • • • Well absorbed after oral administration 100% Bioavailability Metabolized via CYP450 2D6 (minor) T ½ : 70 hours Time to peak: 3-4 hours Excreted via kidneys (57%) and intestine (15%) 71 Donepezil (Aricept®) • Initiate at 5 mg QHS and increase to 10 mg after 4 – 6 weeks – May take with or without food • Increase to 23 mg after minimum of 3 months on 10 mg • Special Populations – Low body weight; decreased clearance = increased AEs • Incidence of weight loss increased at dose of 23 mg • Aricept ODT is bioequivalent to regular tabs 72 Donepezil Efficacy 73 Galantamine (Razadyne®) • • • • Indicated for mild & moderate AD Readily crosses BBB with little effect in periphery Similar cognitive improvement as Aricept AE profile – 6-24% GI upset, insomnia, N&V 74 Galantamine (Razadyne®) • Razadyne (BID); Razadyne ER (Q Day) – Switch from IR tablet to ER capsule; use same daily dose • Dosing – – – – Initial 8 mg/day x 4 weeks Increase to 16mg/day x 4 weeks May increase to 24 mg/day Re-titrate after > 3 days interrupted dosing • Starting dose (8 mg/day) is NOT therapeutic! 75 Galantamine (Razadyne®) • ADME – – – – – – – Rapidly absorbed Food decreases Cmax but not AUC 90% Bioavailability Metabolized via CYP450 (2D6, 3A4) T ½ : 7 hours Time to peak: 1 hour (I.R.), 4 hours (E.R.) 25% excreted via kidneys 76 Galantamine (Razadyne®) • Special Populations – Moderate renal or hepatic impairment max dose = 16 mg/day – Severe renal or hepatic impairment; use not recommended • Recommended to take with food and plenty of fluids • Name confusion Razadyne® vs. Rozerum® 77 Galantamine Efficacy 78 Rivastigmine (Exelon®) • Indicated for mild, moderate, and severe AD and PD dementia • Available as oral or patch • Similar efficacy to other cholinesterase inhibitors • AE profile – 7-47% GI upset, N&V, diarrhea – CNS - dizziness, headache, tremor • Dosing – 1.5mg BID to max. 6mg BID – 4.6 mg, 9.5 mg, or 13.3 mg per 24hr patch 79 Rivastigmine (Exelon®) • Dosing – PO: • • • • 1.5mg BID x 4 weeks then 3 mg BID x 4 weeks then 4.5 mg BID x 4 weeks then 6 mg BID (max dose) – Patch: • 4.6 mg, 9.5 mg, or 13.3 mg per 24hr patch • Increase dose after 1 month – Converting from PO to Patch • < 6 mg/day 4.6 mg/24h • 6-12 mg/day 9.5 mg/24h • Apply patch on the day following the last PO dose 80 Rivastigmine (Exelon®) Patch • Indicated for mild to moderate (9.5 & 13.5 mg) and severe (13.5 mg) dementia – If dose interrupted for > 3 days then re-titrate from 4.6 mg • Special populations – Mild to moderate hepatic impairment & Low body weight • consider 4.6 mg for both initial and maintenance dose • Apply to clean, dry, hairless skin on upper or lower back every 24 hrs (rotate sites) • Application site reactions (erythema / pruritis) are common (6-12%) 81 Rivastigmine (Exelon®) • ADME – Rapidly absorbed – 90% Bioavailability – Metabolized via hydrolysis by acetylcholinesterase – Time to peak: 1 hour (oral), ~8 hours (patch) – 97% excreted via kidneys 84 Take Home Message on Cholinesterase Inhibitors • No real difference in efficacy between drugs • Drug use determined on tolerability, price, etc • Tacrine not on the market any longer • Improvements in cognition modest • Do not alter course of disease 85 Counseling points on Cholinesterase Inhibitors • Use as directed • Adverse Events – Inform patients/caregivers of potential for nausea, vomiting, & diarrhea with potential for dehydration • Especially upon initiation and dose increase – Skin reactions with Exelon patch • Do not use with other cholinergic drugs (e.g., other AChEIs) • Variable efficacy, but does not cure AD • Anticholinergics recommended to be avoided 86 Principles for ChEIs NMDA Antagonists • Memantine (Namenda®) – only FDA approved NMDA antagonist for AD • Believed to work by blocking action of Glutamate at NMDA (NMethyl-D-Aspartate) receptors • NMDA receptors involved in memory and learning • Glutamate excitotoxicity possibly involved in pathogenesis of AD • Memantine shown to modestly improve cognition 88 Memantine (Namenda®) – Glutamate excitatory neurotransmitter – Over activation of NMDA receptor results in excitotoxicity – Memantine binds to Mg2+ site in ion channel – it is an uncompetitive inhibitor – Causes ion channel pore to be closed to Ca2+ influx NMDA Receptor 89 Memantine (Namenda®) • Approved for moderate to severe AD • Shown to modestly slow cognitive decline in AD patients • Used as monotherapy or with ChE Inhibitor • Well tolerated – no significant AEs – H/A, dizziness, sedation, agitation, constipation 90 Memantine (Namenda®) Dosing IR tabs: • 5 mg Q Day x > 1 week, then • 5 mg BID x > 1 week, then • 5 mg Q AM and 10 mg Q PM x > 1 week, then • 10 mg BID Dosing ER caps: • 7 mg Q Day x > 1 week, then • 14 mg Q Day x > 1 week, then • 21 mg Q Day x > 1 week, then • 28 mg Q Day Switching from IR to ER: • Begin ER product day after last dose of IR • If on 10 mg BID 28mg Q Day 91 Memantine (Namenda®) • ADME – T1/2: 60-80 hours – Time to peak: 3-7 hours – 82% excreted via kidneys • Place in Therapy: – Improvements (small) in cognition – Improvements in ADLs – Improvements in behaviors 92 Principles for memantine Other approaches - MAOIs • Theoretical based on MOA – Norepinephrine decreased in AD patients – Inhibition of MAO results in increase norepinephrine centrally • Selegiline – Some trials showed positive outcomes using selegiline • Improvements in memory and attention – Other studies showed no significant improvements 94 Other approaches – Anti-inflammatory Agents • Theoretically could help due to involvement of inflammatory mediators • Studies suggest protective effect • Clinical studies less positive – Prednisone, Diclofenac, Indomethacin all negative • Prednisone associated with decline in cognitive symptoms and behavior • NSAIDs associated with risks but no benefits 95 Other approaches - Estrogens • Increases cerebral blood flow • Neuroprotective • Epidemiological studies suggest lowers risk • Clinical trials negative – WHIMS – Estrogen increased dementia • Estrogen risks e.g., stroke 96 Other approaches – Lipid Lowering Agents • Link between AD risk and cholesterol • Epidemiological studies suggest lower AD in those taking statin – Only for lovastatin and pravastatin • In one study simvastatin showed decrease in βAmyloid plaques in patients with mild AD • No positive clinical studies linking improvement in cognition with statins 97 Other approaches - Vitamin E • Oxidative Stress and Free Radical possibly involved in AD • Vitamin E is an antioxidant • Single prospective clinical study showed a positive outcome for Vitamin E • Conflicting data from epidemiological studies • Vitamin E also associated with risks (although minimal) • Fatigue, GI upset • Increased risk of bleeding if taken with NSAID/ASA • Doses above 400units/day should be avoided if possible 98 Other approaches – Ginko Biloba • A number of studies show mild improvement in cognitive function in demented patients – One study showed Ginko 160mg QD comparable to donepezil 5mg QD • Has been shown to improve cognition in patients suffering MCI and in young to middle aged individuals • Possible MOA: – Increases cerebral blood flow – Acts as antioxidant – neuroprotective – Anti-inflammatory properties • Adverse effects rare – GI upset • Use cautiously in patients taking NSAIDS/ASA 99 Potential Treatments in Research • γ-Secretase Inhibitors – e.g. Semagecestat – Phase III – IDENTITY Study showed negative outcome • Anti β -Amyloid Agents – e.g. Solanezumab – Currently Phase III – EXPEDITION Study 100 Potential Treatments in Research • Sleep? – Melatonin, Ramelteon (Rozerem) • Females more vulnerable? – Decrease in function 2x as fast as males • • • • • • Beta-amyloids- vaccines Tau proteins - vaccines Inflammation – NSAIDs failed Insulin Resistance Brain Imaging and Biomarkers - * Genetics - * 101 TREATMENT OF BEHAVIORAL & PSYCHOTIC SYMPTOMS OF DEMENTIA (BPSD) Behavioral Symptoms • Affects up to 90% – Not included in defining criteria of dementia in current classifying systems • Cause disability, patient distress, caregiver burden, institutionalization • Can be annoying / disruptive threatening / dangerous Behavioral Symptoms • Spectrum of behaviors – – – – – Apathy Wandering Agitation Verbal and/or physical aggression Psychotic symptoms • Evaluate any change in behavior – Meds – Pain – Infection • Evaluate triggers contributing to behaviors – Hunger, cold, pain • Non-pharmacological interventions 1st line Non-Pharmacologic Interventions • • • • • • • Music Aromatherapy Bright lights Massage / touch Validation Reminiscence Establish routine for meals, bedtime, etc. Non-Pharmacologic Interventions • Modify environment (reduce stimulation) – Loud or violent TV, clutter • Exercise – Maintains ambulation & improves mood • Intervene early • Remain calm / voice tone / eye contact • Use visual cues or barriers to discourage wandering Non-Pharmacologic Interventions • • • • • • • Break tasks into simple steps Distractions Give choices in clothing Calendars & clocks to orient to time Gates / locks on doors Grab bars @ toilet and shower Nightlights Depression • Affects up to 50% of patients • Symptoms masked by dementia • Manifests differently in AD patients – Irritability / anxiety / functional decline • Somatic concerns, fear, worry – Dysphoria at earlier stages – Agitation / apathy / motor function decline at later stages • Non Pharm Tx: – Exercise – Increase pleasurable activities (art, writing, music, gardening) Agitation • Complex issue r/t assessment and intervention – Ex. Agitation vs. anxiety • Vague term • “Inappropriate verbal, vocal, or motor activity that is not judged by an outside observer to result directly from the needs or confusion of the agitated individual.” - Cohen-Mansfield, 2010 Agitation • Can include aberrant motor behavior – Wandering – Repetitive, purposeless behaviors – Socially inappropriate activities (ex. sexual behaviors) • Must rule out: Hunger / cold / hot / discomfort / pain, etc. • Modify environment • Pharm Tx: PRN medications Wandering • Identify triggers (boredom, hunger, thirst, etc.) – What’s the goal of wandering for patient? – Searching for something? • • • • Non-Pharm Tx: Music therapy, PT Exercise / redirection Not likely to respond to meds unless 2’ anxiety Burn calories will need increased intake Wandering • Cover / disguise doors and exits • Locks on doors • Alzheimer’s Association MedicAlert + Safe Return • GPS tracker / band Physical and/or Verbal Agitation • Cohen-Mansfield: Four categories of agitation: 1. 2. 3. 4. Physically non-aggressive behavior Verbally non-aggressive behavior Physically aggressive behavior Verbally aggressive behavior • Many times aimed towards caregiver • Threshold: – – – – Harmful to self or others Interferes with functioning Cause disability Impede care Sleep Disturbances • Prevalent in dementia patients • Can include: hypersomnia, insomnia, sleepwake cycle reversal, fragmented sleep, daytime napping, night-time awakenings Sleep Disturbances • Assessment: – Depression – Fear – Pain – Meds that insomnia / incontinence • Interventions: – Adult day care – Warm milk / high carb snack – Melatonin Sleep Disturbances • Sleep Hygiene: – Bedroom free of distractions – Limit naps – Exercise in am or early pm – Limit caffeine / nicotine / extra fluids in pm – Dressed in daytime clothing – Night light for safety Psychotic Symptoms • Delusions (fixed false beliefs) • Less complex than in non-demented patients • Typically involves suspiciousness, abandonment, misidentification – People breaking in – Family poisoning patient – Conspiracy to take $$ / abandon pt Psychotic Symptoms • • • • Hallucinations vs. Perceptual Differences Visual / auditory / tactile hallucinations Hallucinations more common with Lewy Body Rule out other causes – Hearing aids – MRPs – Infections AD vs. Vascular Dementia Alzheimer’s Dementia Vascular Dementia • Similar rates of • Higher prevalence / severity psychotic sx of depression • Slow, steady decline • Less aberrant motor behaviors • Executive functioning more impaired • Patient aware of deficits • Gait disorders Dementia w/Lewy Bodies • 15-25% of dementias – DA and ACH deficits • DA loss EPS / Parkinsonian symptoms – More delusions – Fluctuating cognition – Hallucinations (detailed visual hallucinations) • Occur early in disease Fronto-Temporal Lobe Dementia • AKA: Pick’s Disease • See changes in behavior/personality rather than in cognitive function • Dramatic change in personal/social behavior • Neglect of personal hygiene • Loss of basic emotions • Hyperorality (gluttony, excessive smoking, altered food preferences – ex. sweets) • Pacing • Reduced speech (some patients become mute) PHARMACOLOGIC INTERVENTIONS FOR BPSD Alzheimer's Drugs • ChEI – Modest effect on neuropsychiatric symptoms – Apathy, depression, aberrant motor behaviors most likely to improve • Memantine – Agitation & irritability most likely to improve General Principles • Treating symptom of a disease / will not modify disease • Monitor for adverse effects • Use lowest dose possible • Select meds with “clean” S/E profile OR use S/E profile to benefit Benzodiazepines • Targeted Behaviors: Anxiety / Insomnia / Acute Agitation • Avoid using, if possible. • Use PRN at 1/3 to 1/2 usual dose • Use agents with shorter t ½ • S/E: increased cognitive impairment, fall risk, paradoxical agitation • Avoid EtOH Antidepressants • Targeted Behavior: Depression / Agitation / Insomnia • SSRIs: – Mx CP450 drug interactions – S/E: Activation / sedation / insomnia / dizziness • Use S/E to “benefit” (ex. sedation) • TCAs: – Avoid 2’ S/E profile Antidepressants • SNRIs: – Avoid if s/e are problematic with patient hx • Mirtazapine: – Promotes sleep @ lower doses – Can improve appetite, weight gain • Trazodone: – Useful @ HS sedation or in daytime if agitation Sedative / Hypnotics • Zaleplon (Sonata) / zolpidem (Ambien) ramalteon ( Rozerem) • Can be used safely in elderly • Monitor for daytime sedation, dizziness, falls Diphenhydramine • • • • Avoid use for anxiety, sleep, etc. Beer’s Criteria Drug-drug interaction (with ChEIs) Drug-disease interaction (w AD) Atypical APs • Targeted Behavior: Psychotic symptoms, aggressive / combative behaviors • NOT FDA approved • Use when all other options tried / failed • Black Box Warning: Increased risk of stroke / death in dementia patients • S/E: EPS and TD (less than with typicals) Guideline for Alzheimer’s Disease Management. CA workgroup on Guidelines for Alzheimer’s Disease Management From: Efficacy and Comparative Effectiveness of Atypical Antipsychotic Medications for Off-Label Uses in Adults: A Systematic Review and Meta-analysis JAMA. 2011;306(12):1359-1369. doi:10.1001/jama.2011.1360 Figure Legend: Total global scores are presented and include the symptoms of delusion, hallucination, dysphoria, anxiety, agitation or aggression, euphoria, disinhibition, irritability, apathy, aberrant motor activity, and behavioral disturbances. Weights are from a random-effects analysis. The size of the data markers is proportional to the sample size of the trial. aThe data used for this study were abstracted from the meta-analysis by Schneider et al. From: Efficacy and Comparative Effectiveness of Atypical Antipsychotic Medications for Off-Label Uses in Adults: A Systematic Review and Meta-analysis JAMA. 2011;306(12):1359-1369. doi:10.1001/jama.2011.1360 From: Efficacy and Comparative Effectiveness of Atypical Antipsychotic Medications for Off-Label Uses in Adults: A Systematic Review and Meta-analysis JAMA. 2011;306(12):1359-1369. doi:10.1001/jama.2011.1360 Typical APs • Avoid 2’ cardiac / EPS / TD – TD seen in 50% of elderly after 2 years use Guideline for Alzheimer’s Disease Management. CA workgroup on Guidelines for Alzheimer’s Disease Management Mood Stabilizers / AEDs • Targeted Behaviors: Delusions / Hallucinations / Psychomotor agitation / Combativeness • Not FDA approved • When used with atypical APs, may allow for dose reductions • Treat to response of symptoms • No “therapeutic” level, but monitor if risk of toxicity Guideline for Alzheimer’s Disease Management. CA workgroup on Guidelines for Alzheimer’s Disease Management Behavioral Symptoms • Affects up to 90% – Not included in defining criteria of dementia in current classifying systems • Cause disability, patient distress, caregiver burden, institutionalization • Can be annoying / disruptive threatening / dangerous Behavioral Symptoms • Spectrum of behaviors – – – – – Apathy Wandering Agitation Verbal and/or physical aggression Psychotic symptoms • Evaluate any change in behavior – Meds – Pain – Infection • Evaluate triggers contributing to behaviors – Hunger, cold, pain • Non-pharmacological interventions 1st line