* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File - Wk 1-2
High-altitude adaptation in humans wikipedia , lookup
Organisms at high altitude wikipedia , lookup
Intracranial pressure wikipedia , lookup
Cardiac output wikipedia , lookup
Acute respiratory distress syndrome wikipedia , lookup
Hemodynamics wikipedia , lookup
Haemodynamic response wikipedia , lookup
Biofluid dynamics wikipedia , lookup
Homeostasis wikipedia , lookup
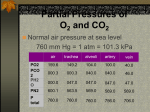
BIOCHEMISTRY AND PHYSIOLOGY OF GAS EXCHANGE 1. Outline respiratory gas exchange and gas transport in blood, including describing effects of shunts. GAS LAWS: General Gas Law – the pressure of a gas in inversely proportional to its volume (at a constant temp this is called Boyle’s Law) P = nRT V o Air flows from areas higher to lower pressure. When alveolar volume increases, causing pleural pressure to decrease below atmospheric pressure air moves INTO the lungs. When alveolar volume decreases, causing pleural pressure to increase above atmospheric pressure air moves OUT of the lungs. Dalton’s Law - the partial pressure of a gas in a mixture of gases is the % of the gas in the mixture times the total pressure of the mixture of gas. o Gases move from areas of higher to areas of lower partial pressure. The greater the difference in partial pressures between two points, the greater the rate of gas movement. Maintaining partial pressure differences ensures gas movements. Henry’s Law – the concentration of a gas dissolved in a liquid is equal to the partial pressure of the gas over the liquid times the solubility coefficient of the gas. o Only a small amount of the gases in air dissolves in the fluid lining the alveoli. Partial Pressure At sea level, atmospheric pressure is approx. 760 mmHg i.e.: the mixture of gases that constitute atmospheric air exert a total pressure of 760 mmHg. The major components of dry air are Nitrogen (79%) and Oxygen (21%). The Partial Pressure is the pressure exerted by each type of gas in a mixture Dalton’s Law: e.g. Nitrogen = 78.62% of the volume of atmospheric air so the partial pressure resulting from nitrogen is 0.7862 x 760 mmHg. Therefore PN2 = 597.5mm Hg. e.g. Oxygen = 20.84% of the volume of atmospheric air so the partial pressure resulting from oxygen is 0.2084 x 760 mmHg. Therefore PO2 = 158.4 mm Hg Partial pressure of respiratory gases as they enter and leave the lungs (at sea level) Atmospheric air Humidified air Alveolar Air Expired Air N2 597.0 (78.62%) 563.4 (74.09%) 569.0 (74.9%) 566.0 (74.5%) O2 159.0 (20.84%) 149.3 (19.67%) 104.0 (13.6%) 120.0 (15.7%) CO2 0.3 (0.04%) 0.3 (0.04%) 40.0 (5.3%) 27.0 (3.6%) H2O 3.7 (0.50%) 47.0 (6.20%) 47.0 (6.2%) 47.0 (6.2%) Total 760.0 (100%) 760.0 (100%) 760.0 (100%) 760.0 (100%) Diffusion of Gases through Liquids When a gas comes into contact with a liquid such as water, it tends to dissolve in the liquid. At equilibrium, the [ ] of a gas in the liquid is determined by its partial pressure in the gas and its solubility in the liquid (Henry’s Law). [ ] of dissolved gas = (partial pressure of gas) x (solubility coefficient) Solubility coefficient = a measure of how easily the gas dissolves in the liquid. CO2 however is 24 times more soluble than O2; therefore, CO2 passes out through the respiratory membrane more readily than O2 enters. Gases don’t usually produce a partial pressure in a liquid as they do when they’re in a gaseous state, however the [ ] of a gas in a liquid can be calculated using the general gas law equation. Diffusion of gases through the respiratory membrane The factors influencing the rate of gas diffusion across the respiratory membrane include: 1) The membrane thickness 2) Diffusion coefficient 3) Membrane surface area 4) Partial pressure difference 1) Respiratory membrane thickness ↑thickness → ↓ rate of diffusion. Disease such as pulmonary oedema, TB and pneumonia are the most common causes of ↑ membrane thickness. 2) Diffusion coefficient Is a measure of how easily the gas diffuses through a liquid or tissue. This is approximately the same as the diffusion coefficient for the gas through water i.e diffusion coefficient of O2 is 1 then the relative diffusion coefficient of CO2 is 20, meaning CO2 diffuses through the respiratory membrane 20 times more rapidly than oxygen does. When the respiratory membrane becomes damaged through disease, the capacity for allowing movement of O2 and CO2 is impaired. 3) Membrane Surface Area In a healthy adult, the total surface area of the respiratory membrane is approx. 70m2. Emphysema and lung cancer cause a ↓ in the respiratory membrane surface area. Pneumonia and pulmonary oedema are more acute conditions which cause the alveoli to fill with fluid thus ↓ing the SA available for gas exchange. 4) Partial Pressure Difference The partial pressure difference of a gas across the respiratory membrane is the difference between the partial pressure of the gas in the alveoli and the partial pressure of the gas in the blood of the pulmonary capillaries. Net diffusion occurs from higher to lower partial pressures. Normally PO2 is greater in the alveoli then in the blood of the pulmonary capillaries and PCO2 is greater in the blood than in the alveolar air. By ↑ing alveolar ventilation, the partial pressure difference for O2 and CO2 can be raised. The greater volume of atmospheric air exchanged with the residual volumes raises alveolar PO2, lowers PCO2 thus promoting gas exchange. Conversely, inadequate ventilation causes a lower-than-normal partial pressure difference for O2 and CO2 resulting in inadequate gas exchange. Relationship Between Ventilation and Pulmonary Capillary Blood Flow The normal relationship between ventilation and pulmonary capillary flow can be disrupted in 2 ways: 1. When ventilation exceeds the ability of the blood to pick up oxygen (i.e. with ↓ CO after a heart attack) 2. When ventilation is not great enough to provide the oxygen needed to oxygenate the blood flowing through the pulmonary capillaries (i.e. asthma → bronchiole constriction → ↓ air delivery to the alveoli) Blood that isn’t completely oxygenated is called shunted blood. 2 sources of shunted blood exist in the lungs: Anatomic shunt – results when deoxygenated blood from the bronchi mixes with blood of the pulmonary veins. The other source of shunted blood is blood that passes through pulmonary capillaries but doesn’t become fully oxygenated Physiologic shunt – is the combination of deoxygenated blood from the anatomic shunt and pulmonary capillaries. Normally 1-2% of CO passes through the physiologic shunt. When standing, blood flow and ventilation is greater at the base of the lung than at the top due to gravity. The arterial pressure is therefore also greater at the base of the lung (due to hydrostatic pressure caused by gravity) resulting in increased blood flow and distended blood vessels. Oxygen Diffusion Gradients PO2 within the alveoli is ~104 mmHg and as the blood flows into the pulmonary capillaries, it has a PO2 of ~40 mmHg. So O2 diffuses from the alveoli into the pulmonary capillaries (due to the pressure difference). By the time the blood has reached the venous end of the pulmonary capillaries, equilibrium is achieved and the PO2 of the blood is 104 mmHg – even with exercise. So the blood leaving the pulmonary capillaries has a PO2 of 104 mmHg, but the blood leaving the lungs in the pulmonary veins has a PO2 of ~95mmHg. This ↓ is due to the blood from the pulmonary capillaries mixing with deoxygenated (shunted) blood from the bronchial veins. Blood entering the arterial end of tissue capillaries has PO2 of ~ 95mmHg. PO2 of interstitial spaces is only ~40mmHg and ~20mmHg in individual cells. Thus O2 diffuses from the tissue capillaries → interstitial fluid → the cells of the body to be used for anaerobic metabolism. Carbon Dioxide Diffusion Gradients CO2 is continuously produced as a by-product of cellular respiration and a diffusion gradient is established from tissue cells to the blood within the tissue capillaries. Intracellular PCO2 is ~46mmHg Interstitial fluid PCO2 is ~45mmHg Tissue capillary (Arterial end) PCO2 is ~ 40mmHg Tissue capillary (Venous end) PCO2 is ~45mmHg As blood flows through the tissue capillaries, CO2 diffuses from a higher PCO2 to a lower PCO2 until equilibrium is reached. After blood leaves the venous end of capillaries, it goes through the CVS to the lungs. Pulmonary capillaries (Arterial end) PCO2 is ~45mmHg Alveolar PCO2 is ~40mmHg Pulmonary capillaries (Venous end) PCO2 is ~ 40mmHg CO2 therefore diffuses from the capillaries into the alveoli. HAEMOGLOBIN AND OXYGEN TRANSPORT ~98% of O2 transported in the blood is in combination with Hb in RBCs with the remaining 2% dissolved in the water part of the plasma. The O2-Hb combination is reversible. In the pulmonary capillaries, O2 binds to Hb and in the tissue spaces O2 diffuses away from the Hb and enters the tissues. Effect of PO2 The O2-Hb dissociation curve represents the % of Hb saturated with O2 at any given PO2. Hb is saturated when an O2 is bound to all 4 heme groups. At any PO2 above 80mmHg, ~95% of the Hb is saturated. When tissues O2 needs ↑, blood PO2 ↓ and more O2 is released for use by the tissues. Effect of pH, PCO2 and Temp As blood pH ↓’s (due to↑ H+ ions), the amount of O2 bound to Hb at any given PO2 also ↓’s. This is because the H+ ions combine with the protein part of the Hb molecule and change its 3D structure causing a ↓ ability to bind with O2. Conversely, as the blood pH ↑’s, the Hb has an ↑’d ability to bind to O2. The effect of pH (H+ ions) on the O2-Hb dissociation curve is called the Bohr Effect. ↑ PCO2 → ↓ ability of Hb to bind to O2 due to the effect of Co2 on pH. As CO2 levels ↑, more H+ ions are produced → ↓ pH. As CO2 levels ↓, less H+ ions produced → ↑ pH. Blood passing through tissue capillaries picks up CO2. Hence the blood CO2 levels ↑, Hb has a ↓ affinity for O2 and a greater amount of O2 is released in the tissue capillaries than would be released if CO2 wasn’t present. When blood is returned to the lungs and passes through the pulmonary capillaries, CO2 leaves the capillaries and enters the alveoli. As a consequence, CO2 levels in the pulmonary capillaries are ↓ and the affinity oh Hb for O2 ↑’s. ↑ temp also → ↓ tendency for O2 to remain bound to Hb. Hence, the ↑ amount of O2 released into the tissues with ↑ metabolism (e.g. during exercise). This causes a shift to the RHS. As much as 75-85% of the oxygen is released into the tissues from the Hb. In the lungs, the curve shifts to the LHS due to the lower levels of CO2, lower temp and lower lactic acid levels. The affinity of Hb for O2 therefore ↑’s and is easily saturated. Effect of BPG As RBCs break down glucose for energy, they produce 2,3-biphosphoglycerate. BPG binds to Hb and ↑’s its ability to release O2. ↑ BPG → Hb releasing more O2 ↓ BPG → Hb releasing less O2 e.g. people living at high altitudes have ↑ levels of BPG which ↑’s oxygen delivery to tissues by causing Hb to release more oxygen. FETAL Hb As fetal blood circulates through the placenta, O2 is released from the mother’s blood and CO2 is released from the fetal blood. Fetal blood is very good at picking up O2 for several reasons: 1. The [ ] of fetal Hb is ~50% higher than the [ ] of maternal Hb 2. Fetal Hb is different from maternal Hb. Its O2-Hb curve is to the LHS of the maternal O2-Hb curve. Thus for a given PO2 fetal Hb can hold more O2 than the mothers. 3. BPG has little effect on fetal Hb. It does not cause it to release O2. 4. The movement of CO2 out of the fetal blood causes the O2-Hb curve to shift to the LHS. At the same time, the movement of the CO2 into the mother’s blood causes her O2-Hb curve to shift to the RHS. Thus the mother’s blood releases more O2 and the fetal blood picks up more O2. This is called the Double Bohr Effect. TRANSPORT OF CARBON DIOXIDE CO2 is transported in the blood in 3 major ways: 70% is transported in the form of bicarbonate ions 23% is transported in combination with blood proteins (mostly Hb) 7% is transported as CO2 dissolved in the plasma The most abundant protein to which CO2 binds in the blood is Hb. It reversibly binds to the globin part of the Hb molecule and many CO2 molecules can bind to a single Hb molecule. Hb that’s released its O2 binds more readily to CO2 than Hb that still has O2 bound to it. This is called the Haldane effect. In tissues, after Hb has released O2 the Hb has an ↑ ability to pick up CO2. In the lungs, as Hb binds to O2 the Hb more readily releases CO2. Carbon Dioxide and Blood pH Blood pH refers to the pH in the plasma NOT inside the RBCs. In plasma CO2 can combine with water to from carbonic acid (catalysed by carbonic anhydrase) on the surface of capillary endothelial cells. The carbonic acid then dissociated to form bicarbonate and H+. Thus, as plasma CO2 levels ↑, H+ levels also ↑ and blood pH ↓’s. Respiration therefore plays an important role in regulating plasma CO2 levels. HYPERventilation → ↓ plasma CO2 levels HYPOventilation → ↑ plasma CO2 levels Chloride Shift ↓ing the amount of bicarbonate and H+ ions inside RBCs promotes CO2 transport bc as these reaction products are removed and their ion [ ]’s ↓, more CO2 combines with water to form additional bicarbonate and H+ ions. In the Chloride Shift bicarbonate ion [ ] inside RBCs and ↓ by exchanging them for Cl- ions through carrier molecules in the membrane. The exchange of –ve ions maintains electrical balance between RBCs and the plasma. Hb which binds H+, decreases the [H+] inside the RBCs – thus functioning as a buffer by resisting a pH rise within the RBCs. In the lungs the reverse happens. Co2 diffuses from the RBCs into the alveoli. As the CO2 levels in the RBCs ↓, carbonic acid is converted to CO2 and water. In response, bicarbonate ions join with the H+ to form carbonic acid. As the bicarbonate and H+ ions decree bc of this reaction, they are replaced. Bicarbonate ions enter the RBC in exchange for Cl- ions and H+ ions are released from Hb. SHUNTS A passage connecting two chambers or vessels diverting blood from one to the other PATHOLOGICAL shunts are abnormal communication between chambers or blood vessels They occur as L → R shunts (e.g. patent ductus arteriosus) and R → L shunts (e.g. tetralogy of fallot) In respiration can also have a physiological shunt with a V/Q mismatch In R → L shunts, deoxygenated blood moves from the pulmonary circulation into the systemic circulation missing out on gas exchange (e.g. septal defect deoxygenated blood goes straight into systemic circulation by passing from the RV into the LV) mixing of blood causes less oxygen to be in blood and can often see cyanosis. In L → R shunts, don’t often see cyanosis till later in life and is usually associated with heart failure Recirculation of blood through lungs causes increased load for the LV, pulmonary oedema and pulmonary congestion Can be difficult to get enough blood to systemic circulation with exercise Not enough PO2 systemically although will have oxygenated blood going through pulmonary circulation which is a waste of energy.