* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 15-2-3to6大环内酯氨基苷四环素人工合成抗菌药
Marine microorganism wikipedia , lookup
Gastroenteritis wikipedia , lookup
Human microbiota wikipedia , lookup
Traveler's diarrhea wikipedia , lookup
Staphylococcus aureus wikipedia , lookup
Infection control wikipedia , lookup
Antimicrobial surface wikipedia , lookup
Bacterial cell structure wikipedia , lookup
Antibiotics wikipedia , lookup
Carbapenem-resistant enterobacteriaceae wikipedia , lookup
Neonatal infection wikipedia , lookup
Bacterial morphological plasticity wikipedia , lookup
Urinary tract infection wikipedia , lookup
Anaerobic infection wikipedia , lookup
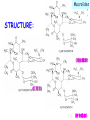
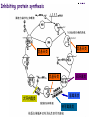
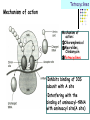
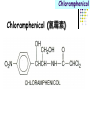
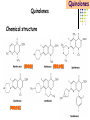
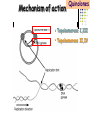
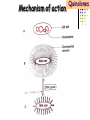
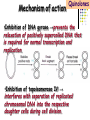
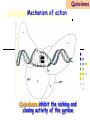
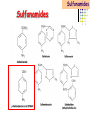
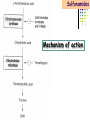
Section 15. Infection disease and Anti-infective drugs (第十五篇 感染性疾病与抗感染药) 第二部分 content Part 3. Macrolides(大环内酯类), Lincomycin(林可霉素类), and Vancomycin(万古霉素) Part 4. Aminoglycosides(氨基糖苷类) and Polymyxins(多黏菌素类) Part 5. Tetracyclines(四环素类) and Chloramphenicol(氯霉素) Part 6. Synthetic antimicrobial agents(人工 合成抗菌药) 细菌蛋白质的合成的简介 3 细菌蛋白质的 合成的简介 30S 30S 1 3 2 1 21 aa1-tRNA 3 1 P 2 3T 1 3 2 1 1 R 3 2 1 A 50S 3 2 T mRNA mRNA 31 3TmRNA 2T TmRNA aa aa32-tRNA -tRNA A P 50S Part 3 Macrolides(大环内酯类), Lincomycin(林可霉素类), and Vancomycin(万古霉素) Macrolides History 1952 Erythromycin(红霉素) 1970s Acetylspiramycin(乙酰螺旋霉素) Medecamycin(麦迪霉素) josamycin(交沙霉素) 1980s Clarithromycin (克拉霉素) Roxithromycin(罗红霉素) Azithromycin(阿奇霉素) Macrolides 14碳环大环内酯类: 16碳环大环内酯类: 红霉素(erythromycin) 克拉霉素 (clarithromycin) 罗红霉素 (roxithromycin) 15碳环大环内酯类: 阿奇霉素 (azithromycin) 吉他霉素(kitasamycin) 交沙霉素(josamycin) 乙酰螺旋霉素 (acetylspiramycin) 麦迪霉素(medecamycin) Macrolides STRUCTURE: (克拉霉素) (红霉素) (阿奇霉素) First generation Macrolides Erythromycin (红霉素) Antimicrobial activity Gram-positive organisms: pneumococci(肺炎双球菌 ), streptococci(链球菌), staphylococci(葡萄球菌) , diphtheriae (白喉)etc Gram-negative organisms:legionella(军团菌),bacillus pertussis(百日咳), brucella(布氏) , meningococci(脑膜炎球菌), diplococcus gonorrhoeae (淋 病双球菌) etc Others: mycoplasma(支原体), chlamydia trachomatis(沙眼衣 原体), rickettsia(立克次体), spirotchete (螺旋体 ), anaerobes(厌氧菌) etc. Mechanism of action Macrolides Target 50s ribosomal RNA Mechanism inhibition of translocation of mRNA Macrolides ① Chloramphenicol ② Clindamycin Macrolides ③ Tertracyclines Macrolides Pharmokinetics Not stable at acid pH Metabolized in liver Excreted in bile Drugs: erythromycin stearate(硬脂酸红霉素) erythromycin ethylsuccinate(琥乙红霉素,利 君沙) erythromycin estolate(无味红霉素) Macrolides Clinical uses As penicillin substitute in penicillinallergic or resistant patients with infections caused by staphylococci, streptococci and pneumococci Pertussis,diphtheriae Legionella and mycoplasma pneumonia H.p infection Macrolides Mechanism of resistance Modification of the ribosomal binding site Production of esterase that hydrolize macrolides Active efflux system Macrolides Adverse reactions Gastrointestinal effects Liver toxicity Cardiotoxicity Macrolides Second generation Advantage : Broader spectrum, higher activity Orally effective High blood concentration Longer t 1/2 Less toxicity Mainly used in respitory tract infection Macrolides Azithromycin (阿齐霉素,丽珠奇乐) Has the strongest activity against mycoplasma pneumoniae(肺炎支原体) More effective on Gram-negative bacteria Well tolerated T1/2 :35~48h once daily Mainly used in respitory tract infection Macrolides Roxithromycin (罗红霉素,严迪) 1987 France The highest blood concentration F 72%~85% Respiratory tract infection and soft tissue infection Low adverse effects Macrolides Clarithromycin(甲红霉素,克拉霉素) Has the strongest activity on Grampositive bacteria, legionella pneumophila, chlamydia pneumoniae and H.p Good pharmacokinetic property Low toxicity Macrolides Third generation Ketolides(酮基大环内酯类) Ketolides are semisynthetic 14-membered-ring macrolides, differing from erythromycin by substitution of a 3-keto group for the neutral sugar Lcladinose. Telithromycin (泰利霉素) It is active in vitro against Streptococcus pyogenes, S pneumoniae, S aureus, H influenzae, Moraxella catarrhalis, mycoplasmas, Legionella, Chlamydia, H pylori, N gonorrhoeae, B fragilis, T gondii, and nontuberculosis mycobacteria. Many macrolide-resistant strains (macrolides-lincomycinsstreptogramins, MLS)are susceptible to ketolides because the structural modification of these compounds renders them poor substrates for efflux pump-mediated resistance and they bind to ribosomes of some bacterial species with higher affinity than macrolides. Lincomycin & Clindamycin Part 3-2 Lincomycin (林可霉素)and Clindamycin(克林霉素) ① Chloramphenicol ② Clindamycin Macrolides ③ Tertracyclines Mechanism : Binding to 50s ribosome subunit and inhibiting protein synthesis Lincomycin & Clindamycin Antimicrobial activity Gram-positive organisms Bacteroide fragilis and other anaerobes Pharmacokinetics Absorbed well Penetrate well into most tissues including bone Lincomycin & Clindamycin Clinical uses Severe anaerobic infection Acute or chronical suppurative osteomylitis(化脓性骨 髓炎), arthritis caused by susceptive organisms especially Staphylococci aureus(金黄色葡萄球菌) Adverse reactions Gastrointestinal effects: severe diarrhea and pseudomembranous enterocolitis caused by Clostridium difficile(难辨梭状芽孢杆菌): vancomycin & metronidazole(甲硝唑) Impaired liver function , neutropenia(中性粒细胞减少) Vancomycin Part 3-3 ----------last choice Vancomycin (万古霉素) & Teicoplanin(替考拉宁) Vancomycin Vancomycin (万古霉素) Mechanism of action--Inhibit cell wall synthesis -Lactam antibiotics vancomycin transpeptidase Vancomycin Vancomycin(万古霉素) Antimicrobial spectrum: Narrow spectrum, active only against grampositive bacteria paticularly staphylococci Pharmacokinetics Poorly absorbed from intestinal tract, iv Excreted from glomerular filtration 90% Vancomycin Vancomycin(万古霉素) Clinical uses Infection caused by MRSA, MRSE and penicillin-resistant pneumococcus Treatment of antibiotic-associated enterocolitis caused by clostridium difficile po Adverse reaction Ototoxicity & nephrotoxicity Red-man syndrome Vancomycin Teicoplanin(替考拉宁) Similar to vancomycin in mechanism and antimicrobial spectrum Can be given im as well as iv Less adverse reactions Linezolid Part 3-4 Oxazolidinones(恶唑烷酮类) Linezolid (利奈唑胺) Linezolid is a member of the oxazolidinones, a new class of synthetic antimicrobials. Antimicrobial spectrum: Mechanism of action It is active against gram-positive organisms including staphylococci, streptococci, enterococci, gram-positive anaerobic cocci, and grampositive rods such as corynebacteria and Listeria monocytogenes. It is primarily a bacteriostatic agent except for streptococci for which it is bactericidal. There is modest in vitro activity against Mycobacterium tuberculosis. Linezolid inhibits protein synthesis by preventing formation of the ribosome complex that initiates protein synthesis. Its unique binding site, located on 23S ribosomal RNA of the 50S subunit, results in no cross-resistance with other drug classes. Mechanism of Resistance Resistance is caused by mutation of the linezolid binding site on 23S ribosomal RNA. Adverse reaction The principal toxicity of linezolid is hematologic—reversible and generally mild. Thrombocytopenia(血小板减少症) is the most common manifestation (seen in approximately 3% of treatment courses), particularly when the drug is administered for longer than 2 weeks. Neutropenia may also occur, most commonly in patients with a predisposition to or underlying bone marrow suppression. Pharmacokinetics Linezolid is 100% bioavailable after oral administration and has a half-life of 4–6 hours. It is metabolized by oxidative metabolism, yielding two inactive metabolites. It is neither an inducer nor an inhibitor of cytochrome P450 enzymes. Peak serum concentrations average 18 g/mL following a 600 mg oral dose. The recommended dose for most indications is 600 mg twice daily, either orally or intraveneously. Clinical uses Linezolid vancomycin-resistant E faecium infections; nosocomial pneumonia(医院获得性肺炎); community-acquired pneumonia(社区获得性肺炎); skin infections Streptogramins Part3-5 Streptogramins (链阳性菌素) Streptogramins are effective in the treatment of Vancomycin-resistant Staphylococcus aureus (VRSA) and Vancomycin-resistant enterococcus (VRE), two of the most rapidly-growing strains of multidrugresistant bacteria. Members include: Quinupristin/dalfopristin (喹奴普丁-达福普丁 ) Pristinamycin Virginiamycin NXL 103, a new oral streptogramin currently in phase II trials (As of). It will be used to treat respiratory tract infections. Daptomycin Part3-6 lipopeptide antibiotic Daptomycin(达托霉素) Mechanism of action Disruption of the bacterial membrane through the formation of transmembrane channels, resulting in a loss of membrane potential leading to inhibition of protein, DNA and RNA synthesis, which results in bacterial cell death. Antimicrobial spectrum: Daptomycin Daptomycin is unable to permeate the outer membrane of Gram-negative bacteria, thus its spectrum is limited to Gram-positive organisms only. Daptomycin has activity against Staphylococci (including MRSA, VISA, and VRSA), Enterococci (both E. faecalis and E.faecium, including VRE), and Streptococci (including DRSP), as well as most other aerobic and anaerobic Gram-positive bacteria. Polypeptide antibiotics Vancomycin & Teicoplanin Polymyxins bactitracin Polymycins Active only against gram-negative rods, particularly P.aeruginosa Mechanism:increase permeability of cell membrane Mainly used in P.aeruginosa infection when other drugs are resistant Toxicity: nephrotoxicity & neurotoxicity Baciteracin Active against gram-positive bacteria Inhibit cell wall formation No cross-resistance with other agents Topical use only because of nephrotoxicity Part 4 Aminoglycosides(氨基糖 苷类) & Polymyxins(多黏 菌素类) Aminoglycosides (氨基苷类) Summarization of aminoglycosides The aminoglycosides are compounds contanining characteristic amino sugars joined to a hexose nucleus in glycosidic(糖苷) linkage. Most aminoglycosides, which are prepared by natural fermentation from various species of streptomyces, are a group of bactericidal drugs sharing chemical, antimicrobial, pharmacological, and toxic characteristics. Aminoglycosides (氨基苷类) Natural Aminoglycosides 链霉素(streptomycin) 新霉素(neomycin) 妥布霉素(tobramycin) 卡那霉素(kanamycin) 大观霉素(spectinomycin) Semisynthetic Aminoglycosides 阿米卡星 (amikacin) 庆大霉素(gentamicin) 西索米星(sisomicin) 小诺米星(micronomicin) 奈替米星(netilmicin) Structure of streptomycin Structures of several important aminoglycoside antibiotics. Aminoglycosides Spectrum of activity Aminoglycosides are effective against aerobic gram-negative bacteria, especially in bacteremia, sepsis, or endocarditis. Aminoglycosides Mechanism of action The mechanism of Aminoglycosides is to inhibit protein synthesis in susceptible microorganisms by interfering with the initiation complex of peptide formation. inducing misreading of the code on the mRNA template, which causes incorporation of inappropriate amino acid into peptide. by rupturing the polysomes into monosome, which become nonfunctional. Inhibiting protein synthesis 氨基苷类 氨基苷类 氨基苷类 大环内酯类 四环素类 氯霉素类 林可霉素类 Mechanisms of resistance Three principal mechanisms have been established: (1) production of a transferase enzyme or enzymes inactivates the aminoglycoside by adenylylation, acetylation, or phosphorylation. This is the principal type of resistance encountered clinically. (Specific transferase enzymes are discussed below.) (2) There is impaired entry of aminoglycoside into the cell. This may be genotypic, ie, resulting from mutation or deletion of a porin protein or proteins involved in transport and maintenance of the electrochemical gradient; or phenotypic, eg, resulting from growth conditions under which the oxygen-dependent transport process described above is not functional. (3) The receptor protein on the 30S ribosomal subunit may be deleted or altered as a result of a mutation. Aminoglycosides Pharmacokinetics • poorly absorbed from the gastrointestinal tract. • must be given intramuscularly or intravemously for systemic infection. • excreted almost entirely unchanged by glomerular filtration, which is greatly reduced in renal impairment, causing toxic blood levels. Aminoglycosides Adverse effects • Ototoxicity Aminoglycosides are potentially toxic to branches of the eighth cromial nerve. The evidence indicates that the sensory receptor portions of the inner ear ( hair cells of the cochlea) are affected rather than the nerve itself. cochlear damage(耳蜗损伤): Kanamycin>Amikacin> sisomicin>gentamicin>tobramycin vestibular impairment(前庭受损): Kanamycin>Streptomycin>sisomicin> gentamicin> tobramycin Aminoglycosides Nephrotoxicity • Nephrotoxicity may develop during or after use of an aminoglycosides, those elderly, debilitated patients, and patients with preexisting renal dysfunction. • Nephrotoxicity is dose dependent and damage to the proximal tubular epithelium usually begins after five to seven days of therapy. • The toxicity results from accumulation and retention of aminoglycosides in the proximal tubular cells. • The renal injury may lead to acute renal failure. Neomycin>Kanamycin>gentamicin>Streptomycin or tobramycin>Amikacin Aminoglycosides Neuromuscular blockade The aminoglycosides rarely cause neuromuscular blockade that can lead to progressive flaccid paralysis and potentially total respiratory arrest. The risk is greatest after rapid iv administration. Neomycin>Streptomycin >Amikacin or Kanamycin>gentamicin > tobramycin Clinical uses Aminoglycosides are mostly used against gramnegative enteric bacteria, especially when the isolate may be drug-resistant and when there is suspicion of sepsis. They are almost always used in combination with a lactam antibiotic to extend coverage to include potential gram-positive pathogens and to take advantage of the synergism between these two classes of drugs. Penicillin-aminoglycoside combinations also are used to achieve bactericidal activity in treatment of enterococcal endocarditis and to shorten duration of therapy for viridans streptococcal and staphylococcal endocarditis. Which aminoglycoside and what dose should be used depend on the infection being treated and the susceptibility of the isolate. Aminoglycosides Streptomycin (链霉素) Spectrum of activity and therapy of Streptomycin (1) most gram-negative bacilli and some gram-positive cocci. (2) organisms that cause plague(鼠疫) and in combination with penicillin G against bacterial endocarditis. (3) antituberculosis agent. A major disadvantage of streptomycin therapy is the development of frequent bacterial resistance to the drug. Aminoglycosides Untoward effects of streptomycin Hypersensitivity reactions can occur. Labyrinthine damage (迷路破坏) and vestibular disturbances can occur. Streptomycin should not be given with other ototoxic drugs . Renal effects are minimal at normal doses. Neuromuscular junction blockade may occur when streptomycin is given at high doses and in combination with curariform drugs (箭毒样药物). Aminoglycosides Gentamicin used in the treatment of a serious infections caused by a large number of gram-negative organisms. Gentamicin is of the first choice when these infections occurs 1) urinary tract infections, bacteremia resulting from Escherichia coli. 2) bile duct and urinary tract infections caused by proteus mirabilis. Gentamicin Gentamicin It is effective against both gram-positive and gram-negative organisms, and many of its properties resemble those of other aminoglycosides. It is active alone, but also as a synergistic companion with –lactam antibiotics, against pseudomonas, proteus, enterobacter, klebsiella, serratia, stenotrophomonas, and other gram-negative rods that may be resistant to multiple other antibiotics. Like all aminoglycosides, it has no activity against anaerobes. Clinical uses Gentamicin Intramuscular or intravenous administration used in the treatment of a serious infections caused by a large number of gram-negative organisms. Gentamicin is of the first choice when these infections occurs 1) urinary tract infections, bacteremia resulting from Escherichia coli. 2) bile duct and urinary tract infections caused by proteus mirabilis. 3) Gentamicin combined with carbenicillin is of the first choice for the treatment of infected burns, bacteremia, urinary tract infection resulting from pseudomonas aeruginosa. Gentamicin Topical administration Creams, ointments, and solutions containing 0.1–0.3% gentamicin sulfate Clinical use: infected burns, wounds, or skin lesions and the prevention of intravenous catheter infections. 10 mg can be injected subconjunctivally for treatment of ocular infections. Topical gentamicin is partly inactivated by purulent exudates. Gentamicin Intrathecal administration Meningitis caused by gram-negative bacteria has been treated by the intrathecal injection of gentamicin sulfate, 1–10 mg/d. Neither intrathecal nor intraventricular gentamicin was beneficial in neonates with meningitis, and intraventricular gentamicin was toxic, raising questions about the usefulness of this form of therapy. the availability of third-generation cephalosporins for gram-negative meningitis has rendered this therapy obsolete in most cases. Gentamicin Adverse effects of gentamicin Ototoxicity (耳毒性) is the most serious effect.(The incidence of ototoxicity is in part genetically determined, having been linked to point mutations in mitochondrial DNA, and occurs in 1–5% for patients receiving gentamicin for more than 5 days. ) Nephrotoxicity can occur. Hypersensitivity can also occur. Aminoglycosides Tobramycin 妥布霉素 Spectrum of activity of Tobramycin Tobramycin has a spectrum of activity similar to that of gentamicin but may be slightly more effective against pseudomonas(假单胞菌属). Pharmacokinetic properties of tobramycin virtually identical with those of gentamicin. Adverse effects of Tobramycin Ototoxicity Tobramyci n Tobramycin vs Gentamicin Spectrum of activity of Tobramycin Tobramycin has almost the same antibacterial spectrum as gentamicin with a few exceptions. Gentamicin is slightly more active against serratia(沙雷氏菌), whereas tobramycin is slightly more active against pseudomonas (假单胞菌属); Enterococcus faecalis (粪肠球菌)is susceptible to both gentamicin and tobramycin, but E faecium (屎肠球菌)is resistant to tobramycin. Adverse effects of Tobramycin Ototoxicity Nephrotoxic Nephrotoxicity of tobramycin may be slightly less than that of gentamicin, but the difference is clinically inconsequential. Tobramycin more active than gentamicin versus pseudomonas; Tobramycin may also have less nephrotoxicity Tobramyci n Clinical uses Gentamicin and tobramycin are otherwise interchangeable clinically. Tobramycin is also formulated in solution (300 mg in 5 mL) for inhalation for treatment of Pseudomonas aeruginosa lower respiratory tract infections complicating cystic fibrosis. Neomycin & Kanamycin Neomycin & Kanamycin Antimicrobial Activity & Resistance gram-positive and gram-negative bacteria and some mycobacteria. Pseudomonas (假单胞菌属)and streptococci are generally resistant. The widespread use of these drugs in bowel Clinical use : Preparation for elective surgery, resulted in the selection of resistant organisms and some outbreaks of enterocolitis in hospitals. Cross-resistance between kanamycin and neomycin is complete. Neomycin & Kanamycin Clinical Uses Neomycin and kanamycin are now limited to topical and oral use. Neomycin is too toxic for parenteral(肠外) use. parenteral administration of kanamycin has also been largely abandoned. Paromomycin(巴龙霉素):Effective against visceral leishmaniasis when given parenterally, and this serious infection may represent an important new use for this drug. Neomycin & Kanamycin Adverse Reactions All members of the neomycin group have significant nephrotoxicity and ototoxicity. Auditory function is affected more than vestibular function. Deafness has occurred, especially in adults with impaired renal function and prolonged elevation of drug levels. The sudden absorption of postoperatively instilled kanamycin from the peritoneal cavity (3–5 g) has resulted in curare-like neuromuscular blockade (箭毒样神经肌肉阻 滞 )and respiratory arrest. Calcium gluconate and neostigmine can act as antidotes. hypersensitivity: not common, prolonged application of neomycin-containing ointments to skin and eyes has resulted in severe allergic reactions. Neomycin & Kanamycin Kanamycin Kanamycin has a more limited spectrum of activity than Gentamicin has. It is ineffective against Pseudomonas and most gram-positive organisms. Its clinical uses almost replaced by gentamicin. Neomycin & Kanamycin Neomycin新霉素 Spectrum of activity Neomycin has a spectrum of activity similar to that of kanamycin. Untoward effects Renal damage, eighth-nerve damage resulting in nerve deafness. Amikacin Amikacin 阿米卡星, 丁胺卡那霉素 Specturm of activity of Amikacin Amikacin has a spectrum of activity similar to that of Gentamicin but often is reserved for serratia (沙雷菌属)infections or for cases where resistance to Gentamicin has emerged. Clinical use Intravenous; resistant to many enzymes that inactivate gentamicin and tobramycin; higher doses and target peaks and troughs than gentamicin and tobramycin Adverse effects of Amikacin Ototoxicity is the most serious side effect. Spectinomycin(大观霉素 ) Spectinomycin is an aminocyclitol antibiotic that is structurally related to aminoglycosides. It lacks amino sugars and glycosidic bonds. Intramuscular; sole use is for treatment of antibiotic-resistant gonococcal infections or gonococcal infections in penicillin-allergic patients Polymyxins Polymyxins 多粘菌素类 There are five polymyxins. A, B, C, D, E, Polymyxin E, 多粘菌素E (Colistin,抗敌素) is frequently used in clinics. Polymyxins Spectrum of activity Polymyxins are active mainly against gram-negative bacilli, particulary pseudomonas and coliform organisms. Mechanism of action of polymyxins Polymyxins act by attaching to the cell membranes of bacteria and other membranes rich in phosphatidylethanolamin (磷脂酰乙醇胺) and disrupting the osmotic properties and transport mechanisms of the membrane. This results in leakage of macromolecules and death of the cell. Polymyxins Polymyxins Therapeutic uses Infections caused by pseudomonas or coliform bacteria resistant to other antimicrobial drugs. Untoward effects Neurotoxic effects Polymyxin can cause paresthesias, dizziness and incoordination. Nephrotoxic effects Some proteinuria(蛋白尿), hematuria (血尿) are the evidence of tubular injury. Part 5 Tetracyclines (四环素)& Chloramphenicol(氯霉素) Tetracyclines Tetracyclines -Chemical structure •Tetracycline (四环素) Tetracyclines Clinical used tetracyclines: Tetracycline(四环素); Demeclocycline(地美环素, 去甲金霉素); Metacycline(美他环素, 甲烯土霉素); Doxycycline(多西环素, 强力霉素); Minocycline(米诺环素, 美满霉素). (Antimicrobial activity enhanced from up to down) Tetracyclines Tetracyclines -Overview Crude product Tetracycline(四环素),Chlortetracycline (金霉素),Oxytetracycline (土霉素) Semisynthetic derivative Doxycycline(多西环素), Minocycline(米诺环素) Antimicrobial activity Tetracyclines (1) Bacteriostatic (2) Bactericidal (at high concentration ) (3) Minocycline > Doxycycline > Tetracycline Antimicrobial spectrum Broad-spectrum antibiotic (1) Active against a wide range of aerobic and anaerobic gram- positive and gram-negative bacteria. (2) Effective against Rickettsia(立克次体),Coxiella burnetii(螺旋 体),Mycoplasma pneumoniae(支原体),Chlamydia (衣原体), and Plasmodium (疟原虫). (3) They are not active against fungi,virus. Tetracyclines Mechanism of action (1) Enter bacteria by passive diffusion through the protein channel formed by porin proteins of outer cell membrane(G- organisms) and active transport by an energy-dependent system that pumps all tetracyclins across cytoplasmic membrane (G+ organisms) . (2) Inhibit protein synthesis in susceptible microorganisms. (3) Increase the permeability of the cell membrane Mechanism of action Tetracyclines Mechanism of action: ①Chloramphenicol ②Macrolides, Clindamycin ③Tetracyclines •Inhibits binding of 30S subunit with A site •Interfering with the binding of aminoacyl-tRNA with aminoacyl site(A site) Mechanism of resistance Tetracyclines (1) Decreased intracellular accumulation due to either impaired influx or increased efflux by a active transport protein pump. (2) Ribosome protection that interfere with the tetracycline binding to the ribosome. (3) Enzyme inactivation of tetracycline. ADME Tetracyclines (1) Absorption are impaired by food (except doxycycline and minocycline ) Tetracyclines manly differ in their absorption after oral administration. (2) Distributed widely to tissue and body fluid except for CSF. (3) Excreted mainly in bile and urine. (4) Tetracyclines across the placenta and are also excreted in the milk. (5) Bound to- and damage- growing bones and teeth As a result of chelation with calcium Tetracyclines Clinical Uses (1)Rickettsial(立克次体) infections. (2)Mycoplasma(支原体) infections. (3)Chlamydia(衣原体) infection. (4)Leptospira(螺旋体) infection. (5)Bacterial infection. Tetracyclines Adverse reaction (1)Gastrointestinal effects. (2)Superinfections. (3)Deposition of the drugs in growing teeth and bones. (4)Hepatic toxicity and renal toxicity. (5)Photosensitivity. (6)Pseudotumer cerebri(脑假瘤) (7)Vestibular toxicity(前庭反应 ) Tetracyclines Recent research •Tetracycline and its derivatives doxycycline and minocycline were found to have antiinflammatory and anti-apoptotic properties. •Protect mice from brain ischemia, traumatic brain injury, Huntington’s disease, etc. •The mechanism partially though caspase-1, 3, iNOS, COX-2 etc. Tetracyclines Tigecycline(替加环素) A newly approved tetracycline analog, tigecycline, is a glycylcycline(甘氨酰四环素类) and a semisynthetic derivative of minocycline. Tetracyclines Tigecycline, the first glycylcycline to reach the clinic, has several unique features that warrant its consideration apart from the older tetracyclines. Many tetracycline-resistant strains are susceptible to tigecycline because the common resistance determinants have no activity against it. Its spectrum is very broad. Coagulase-negative staphylococci and Staphylococcus aureus, including methicillin-resistant, vancomycin-intermediate, and vancomycin-resistant strains; streptococci, penicillin susceptible and resistant; enterococci, including vancomycin-resistant strains; gram-positive rods; Enterobacteriaceae; multidrug-resistant strains of Acinetobacter sp; anaerobes, both gram-positive and gramnegative; atypical agents, rickettsiae, chlamydia, and legionella; and rapidly growing mycobacteria all are susceptible. Proteus (变形杆菌)and P aeruginosa(铜绿假单胞菌), however, are intrinsically resistant. Tetracyclines Tigecycline, formulated for intravenous administration only, is given as a 100mg loading dose; then 50 mg every 12 hours. As with all tetracyclines, tissue and intracellular penetration is excellent; consequently, the volume of distribution is quite large and peak serum concentrations are somewhat blunted. Elimination is primarily biliary, and no dosage adjustment is needed for patients with renal insufficiency. In addition to the tetracycline class effects, the chief adverse effect of tigecycline is nausea, which occurs in up to one third of patients, and occasionally vomiting. Neither nausea nor vomiting usually requires discontinuation of the drug. Tigecycline is FDA-approved for treatment of skin and skin-structure infection and intra-abdominal infections. Because active drug concentrations in the urine are relatively low, tigecycline may not be effective for urinary tract infections and has no indication for this use. Because it is active against a wide variety of multidrug-resistant nosocomial pathogens (eg, methicillinresistant S aureus, extended-spectrum -lactamase-producing gram-negatives, and Acinetobacter species), tigecycline is a welcome addition to the antimicrobial drug group. Chloramphenicol Chloramphenicol (氯霉素) Chemical structure p 1246 p776pharm Chloramphenicol Antimicrobial activity (1)Chloramphenicol possesses a wide antimicrobial spectrum. (2) Primarily bacteriostatic , although it may be bactericidal to certain species. Chloramphenicol Mechanism of action Inhibit protein synthesis in susceptible bacteria, and to a lesser extent, in mammalian cell 2. Acts primarily by binding reversibly to the 50 S ribosomal subunit Near the site of action of macrolides and clindamycin, which it inhibits competitively). 1. Mechanism of action Chloramphenicol Mechanism of action: ①Chloramphenicol ②Macrolides, Clindamycin ③Tetracyclines Mechanism of Resistance Chloramphenicol (1) Resistance usually caused by a plasmid-encoded acetyltransferaes乙酰 转移酶 that inactive the drugs. (2) The permeability of bacterial cell membrane is changed. Clinical uses Chloramphenicol (1) Bacterial meningitis. (2) Typhoid fever(伤寒) and other types of systemic Salmonella infections. (3) Eye bacterial infection. (4) Anaerobic infection. (5) Rickettsial disease (立克次体病) and brucellosis (布鲁杆菌病), etc. Adverse reactions Chloramphenicol (1)Hematological Toxicity: By dose-related toxic effect that presents anemia, leukopenia and or thrombocytopenia (血小板减少) By special response manifested by aplastic anemia, leading in many cases to fatal pancytopenia (全血细胞减少). (2) Toxicity for newborn infants: Gray baby syndrome(灰婴综合征). (3)other reactions: hypersensitivity reaction, etc. Drugs interactions Chloramphenicol Inhibits hepatic microsomal cytochrome P450 enzyme, and thus may prolong the t 1/2 of drugs that metabolized by this system, e.g. warfarin, phenytoin, etc. Part 6 Synthetic antimicrobial agents(人工合成抗菌药) Classification of Synthetic antimicrobial agents Ⅰ. Quinolones(喹诺酮类); Ⅱ. Sulfonamides(磺胺类); Ⅲ. Other synthetic antimicrobial agents: Trimethoprim (甲氧苄啶) Nitrofurans (硝基呋喃类), etc. Quinolones Part6-1 Quinolones General features Broad antimicrobial activity and are effective after oral administration for the treatment of a wide variety of infectious disease. Relatively few side effects. Microbial resistance to their action does not develop rapidly. Quinolones Part6-1 Quinolones Chemistry •Derived from basic structure of nalidixic acid (萘啶酸)and have substituents at N-1, C-5, C-7, position 8 and a fluorine atom at position 6. •Fluorine at position 6 enhances gyrase inhibition and cell penetration. Quinolones Quinolones Chemical structure (萘啶酸) (环丙沙星) (诺氟沙星) Classification Generation 1 st (1962-1969) 2 nd (1969-1979) 3 rd (1980-1996) 4 th (1997-) Quinolones Examples Nalidixic acid, 萘啶酸 Pipemidic acid 吡哌酸 Cinoxacin 西诺沙星 Norfloxacin 诺氟沙星 Levofloxacin 左氧氟沙星 Ciprofloxacin 环丙沙星 Ofloxacin 氧氟沙星 sparfloxacin 司帕沙星 Grepafloxacin 格帕沙星 Clinafloxacin 克林沙星 Gatifloxacin 加替沙星 Moxifloxacin 莫西沙星 Quinolones Quinolones First-generation agents(1962-1969) Nalidixic acid, 萘啶酸 •The first generation drug of the quinolone antibiotics •Moderate gram-negative activity and minimal systemic distribution Clinical applications • Uncomplicated urinary tract infections Quinolones Quinolones Second-generation quinolones (1969-1979) Pipemidic acid 吡哌酸 Cinoxacin西诺沙星 •Expanded gram-negative activity and atypical pathogen coverage, but limited gram-positive activity. •Most active against aerobic gram-negative bacilli •Ciprofloxacin remains the quinolone most active against Pseudomonas aeruginosa Quinolones Quinolones Second-generation quinolones (1969-1979) •Active against gram-positive and gramnegative bacteria, mycobacteria, mycoplasma支 原体and legionella species军团杆菌属. •Longer half-lives due to slow elimination, distribution into many tissues and body fluids and penetration into human cells. Quinolones Quinolones Third-generation quinolones (1980-1996 ) Norfloxacin 诺氟沙星, Levofloxacin 左氧氟沙星, Ciprofloxacin 环丙沙星, Ofloxacin 氧氟沙星, Sparfloxacin 司帕沙星 •Added potency against gram-negative bacteria, anaerobes and mycobacteria. •Retain expanded gram-negative and atypical intracellular activity but have improved grampositive coverage Quinolones Quinolones Fourth-generation agents (1997- ) Grepafloxacin 格帕沙星,Clinafloxacin 克林沙星, Gatifloxacin 加替沙星,Moxifloxacin 莫西沙星 •Improve gram-positive coverage, maintain gram-negative coverage, and gain anaerobic coverage. Quinolones Quinolones Antimicrobial activity & spectrum (1) Bactericidal and have significant PAE. (2)Excellent activity against aerobic gramnegative bacteria, some agents have activity against Pesudomonas. (3) Several newer agents with improved activity against aerobic gram-positive bacteria. Quinolones Quinolones Antimicrobial activity & spectrum (4) They also are effective against Chlamydia spp.(衣原体), Legionella pneumophila(军团菌) ,anaerobic bacteria, mycobacteria(分枝杆菌). (5) Some agents have limited activity against multiple-resistance strains. (6) Bactericidal concentration≥ bacteriostatic concentration Quinolones Quinolones Mechanism of actions Topoisomerases : enzymes that control and modify the topological states of DNA in cells. • Topoisomerase I, III catalyse merely the relaxation of DNA • Topoisomerase II (DNA gyrase) catalyse the supercoiling of DNA • Topoisomerase IV involved in the separation process of the DNA daughter chains after chromosome duplication. Quinolones Quinolones Mechanism of actions The quinolone antibiotics target bacterial • DNA gyrase (gram-negative bacteria) and • Topoisomerase IV (gram- positive bacteria). Mechanism of action Quinolones • Topoisomerase I,III • Topoisomerase II,IV Mechanism of action Quinolones The function of DNA gyrase is to introduce supercoils into the linear DNA double helix, which results in the highly condensed 3-dimensional structure of the DNA usually present inside the cell. DNA gyrase consists of two proteins (A and B), with the active species being a heterotetramer (A2B2). Mechanism of action Quinolones Mechanism of action Quinolones •The Gyr-A subunits were proposed to initially bind to the double stranded DNA helix. In an ATP-dependent process, described as "intermediate gate opening step", both DNA strands are cleaved at certain 4 base pair staggered sites. •The 5'ends of the DNA chain are thereby bound covalently to Tyrosin122 residues within the Gyr-A subunits. •Gyr-B subunits are probably responsible for the ATPdependent resealing process of the DNA. Mechanism of action Quinolones •Topoisomerase IV: involved in the separation process of the DNA daughter chains after chromosome duplication. •Unlike DNA gyrase, it is unable to catalyse the supercoiling of DNA, merely its relaxation. •The enzyme comprises two subunits, ParC and ParE. •The ParC protein is homologous to the gyrase A protein, while the ParE subunit is homologous to the gyrase B protein. Mechanism of action Quinolones •Inhibition of DNA gyrase →prevents the relaxation of positively supercoiled DNA that is required for normal transcription and replication. •Inhibition of topoisomerase IV → interferes with separation of replicated chromosomal DNA into the respective daughter cells during cell division. Quinolones () 1 (-) 2 3 Model of the formation of negtive DNA supercoils by DNA gyrase The enzyme binds two segments of DNA (1), creating a node of positive(+) superhelix. The enzyme then in-troduces a doublestrand break in the DNA and passes the front segment through the break (2). The break is then resealed (3), creating a negative(-) supercoil. Quinolones inhibit the nicking and closing activity of the gyrase, and also block the activity of toposomer-ase Ⅳ. Quinolones Mechanism of action Quinolones inhibit the nicking and closing activity of the gyrase Quinolones Resistance Mechanism •Intrinsic resistance is rare •With a frequency of about one in 107– 109, especially among staphylococci, pseudomonas, and serratia沙雷(氏)菌. Quinolones Resistance Mechanism (1) Mutation of the gyrA gene that encoded the A subunit polypeptide can confer resistance to these drugs. (2) Mutation of the gene cfxB and nfxB that encoded the porin decreased permeability of cell membrance. (3) The high expression of norA gene (encoded active pump protein) increased drugs efflex by a active transport protein pump. (4) Plasmid mediated resistance. Quinolones ADME of Quinolones (1) Well absorbed after oral administration. (2) Distributed widely in body tissue, even in CSF. (3) Excreted mainly in urine. Routes of elimination differ among the Quinolones. Clinical Uses Quinolones (1)Urinary tract infections. The main indication for quinolones In the treatment of uncomplicated and complicated UTIs, cure rates can exceed 90% and 80%, respectively. Potent agents to use against Haemophilus ducreyi (软性下疳嗜血杆菌) and penicillinsensitive and penicillin-resistant Neisseria gonorrhoeae淋(病双)球菌. Clinical Uses Quinolones (2) GI and abdominal infections. • Excellent in vitro activity against many enteric pathogens, including Escherichia coli 大肠杆菌, Aeromonas气单胞菌属, Shigella志贺 (氏)杆菌, Salmonella沙门氏菌, Campylobacter 弯曲杆菌属, Vibrio弧菌属, and Yersinia species耶尔森菌 • Furthermore, quinolone drug concentrations in feces are exceedingly high. Clinical Uses Quinolones (3) Respiratory tract infections. Have inferior activity against streptococci链球菌and should not be used as primary therapy for common upper respiratory tract infections. Alternatives for treatment of acute exacerbation of chronic bronchitis in patients with obstructive pulmonary disease who are intolerant of or have developed resistance to first-line antibiotics. Clinical Uses Quinolones antibiotics with activity against Streptococcus pneumoniae, Haemophilus influenzae流感(嗜血)杆菌, and Moraxella catarrhalis粘膜炎莫拉菌. (4) Other infections: Bone, joint and soft tissue infections. Adverse reactions Quinolones (1)Gastrointestinal effects. The most common reactions (2)CNS side effects. Penetrate BBB→ GABA↓ (3)Allergic reaction. Skin rashses, itchs, angioneuroedema , etc. Photosensitivity (4)other effects. Cardiac toxicity: Q-T interval↑ Liver and renal injury Adverse reactions Quinolones (4)other effects. Muscle skeletal system Amyasthenia 肌无力, myosalgia肌痛, joint pain and inflammation Increase intracranial pressure in infants Quinolones agents Pipemidic acid (吡哌酸) Norfloxacin (诺氟沙星) Ciprofloxacin (环丙杀星) Ofloxacin(氧氟沙星) Levofloxacin(左氧氟沙星) Lomefloxacin(洛美沙星) Fleroxacin(氟罗沙星) Sparfloxacin(司帕沙星) Quinolones Pharmacokinetic Properties of Fluoroquinolones Quinolones 喹诺酮类研究两大动向 继续研发第四代氟喹诺酮 近年上市的:吉米沙星(gemifloxacin ),巴洛沙星 目前正在研发中的:西他沙星(sitafloxacin ),奥鲁沙 星 (olamufloxacin )。 注意研究结构变幅更大的喹诺酮 近年上市的帕珠沙星(pazufloxacin )、鲁利沙星 (prulifloxacin ) 非氟喹诺酮格林沙星(garenoxacin ) Part6-2 Sulfonamides Sulfonamides Sulfonamides Sulfonamides Antimicrobial activity (1) Sulfonamides have a wide range of antimicrobial activity. G+,G- bacteria, Nocardia 诺卡菌属, Bedsonia trachomatis沙眼衣原体, etc. Enteric bacteria etc. less effective Rickett's organism (2) Sulfonamides exert only bacteriostatic effect. Sulfonamides Mechanism of action Structural analogs and competitive antagonists of para-aminobenzoic acid (对氨基 苯甲酸 PABA) Prevent normal bacterial utilization of PABA for the synthesis of folic acid. Sulfonamides Mechanism of action Sulfonamides Mechanism of Resistance Originate by random mutation and selection or by transfer of resistance by plasmides. Such resistance usually is persistent and irreversible. Sulfonamides Mechanism of Resistance The resistance characterized by: (1)A lower affinity for sulfonamides by the dihydropteroate synthase (2)Decreased cell permeability or active efflux of the drug (3)An alternative pathway to synthesis the essential metabolites (4)An increased production of essential metaboltes Sulfonamides Classification (1) Oral absorbable agents Short-acting agents Medium-acting agents Long-acting agents (2) Oral nonabsorbable agents (3) Topical agents. (4) Combination agents. Clinical uses Sulfonamides (1) systemic infections. cerebral meningitis Tympanitis 中耳炎 Uncomplicated urinary tract infections Combined with TMP in treating complicated urinary tract infections,respiratory infections,GI infections (2) intestinal infections. Sulfasalazine 柳氮磺吡啶 (3) infections of burn and wound. Sulfadiazine sliver(磺胺嘧啶银) Sulfonamides Adverse reactions (1)Urinary tract disturbances (2)Hypersensitivity reaction (3)Hematopoietic system disturbances↓ (4)Kernicterus 脑核黄疸 caused by bilirubin胆红素 replacment (5)Hepatitis (6)GI disturbances Sulfonamides ADME of sulfonamides (1) Approximately 70%-100% of an oral dose is absorbed. (2) Sulfonamides are distributed throughout all tissues of the body, even in CSF Sulfadiazine磺胺嘧啶and sulfisoxazole 磺胺异恶唑, may be effective in meningeal infections . (3) Sulfonamides readily pass though the placenta. Sulfonamides ADME of sulfonamides (4) Sulfonamides are metabolized in the liver by acetylation. (5) Sulfonamides eliminated mainly in the urine as the unchanged drug and metabolic product. In acid urine, the eliminated are insoluble and may precipitate, thus induced renal disturbance. Sulfonamides Drugs interactions Increase the effects of tolbutamide 甲苯磺丁脲, warfarin , trexan甲氨喋呤 The reason is: all sulfonamides are bound in varying degree to plasma protein. Sulfonamides Sulfonamides Agents (1) Oral absorbable agents Short-acting agents Sulfafurazole(SIZ,菌得清) Sulfadimidine,(SN2,磺胺二甲嘧啶) Medium-acting agents Sulfadiazine(SD,磺胺嘧啶) Sulfamethoxazole(SMZ,新诺明) Long-acting agents Sulfamonomethoxine(SMM,磺胺间甲氧嘧啶) Sulfonamides Sulfonamides Agents (2) Oral nonabsorbable agents Sulfasalazine(柳氮磺吡啶 ) (3) Topical agents. Mafenide(SML, 磺胺米窿) Sulfadiazine sliver(磺胺嘧啶银) Sulfacetamide(SA,磺胺醋酰) Sulfonamides Sulfonamides Agents (4) Combination agents. Co-trimoxazole(复方新诺明) Trimethoprim(甲氧苄啶) in combination with Sulfamethoxazole(1:5) exerts a synergistic effects. Pharmcokinetics: The ADME of the two agent is similar. Pharmcodynamics: Co-block essential enzymes of folate metabolism. Sulfonamides Sulfonamides Agents (4) Combination agents. Co-trimoxazole(复方新诺明) Clinical Use Chronic and recurrent infections in the urinary tract Bacterial respiratory infections GI infections (eg. induced by Salmonella) Adverse reaction There is no evidence that co-trimoxazole, when given in recommended dose, induced folate deficiency in normal persons. The main untoward effects is hypersensitive reactions( eg. involve the skin). Other Synthetic antimicrobial Trimethoprim (甲氧苄啶) Dihydrofolate reductase inhibitor Drug resistance occurs easily when used alone Nitrofurans (硝基呋喃类) Nitrofurantoin(呋喃妥因) Bactericidal agent Co A inhibitor→DNA damage Resistance is rare Clinical applications: Urinary tract infections Other Synthetic antimicrobial Trimethoprim(甲氧苄啶) Nitrofurans(硝基呋喃类): Funacillin(呋喃西林) Furantoin(呋喃妥因) Furazolidone(呋喃唑酮, 痢特灵) END OF CLASS