* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Study Guide KEY Exam III F 2012
History of molecular biology wikipedia , lookup
Supramolecular catalysis wikipedia , lookup
Electrochemistry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Peptide synthesis wikipedia , lookup
Protein adsorption wikipedia , lookup
Citric acid cycle wikipedia , lookup
Chemical reaction wikipedia , lookup
Electrolysis of water wikipedia , lookup
Stoichiometry wikipedia , lookup
Rate equation wikipedia , lookup
Metalloprotein wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Chemical equilibrium wikipedia , lookup
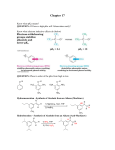
George S. Hammond wikipedia , lookup
Click chemistry wikipedia , lookup
Butyric acid wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Biosynthesis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Petasis reaction wikipedia , lookup
Transition state theory wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Biochemistry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Study Guide KEY Exam III F 2012 Show logic and calculations for all problems. Remember to include units and be careful with sig. fig. Remember a study guide is just a guide for study. It does not necessarily cover every topic that may be on the exam. 0. Which of the following compounds would you predict to have the highest, intermediate, and lowest solubility in water? Explain your answer, using structures where appropriate. H H H C H C C H C O b. a. H H H H H H O C H H c. H C H H H C C H H H C H H Solubility: a > b> c C is a non-polar molecule. It would not be soluble in polar water. Both a. and b. have a non-polar region and a polar region. The non-polar regions have similar surface areas for both molecules. Next, compare the polar regions. a. can form hydrogen bonds with water. Each of its propanol molecules has two H-bonding acceptor sites and 1 donor site. b. can also form hydrogen bonds w/ water. It has two acceptor sites and no donor sites. This has fewer hydrogen bonding sites than molecule a. a. is most soluble. b. has intermediate solubility. c. is least soluble. Drawings follow. Indicates hydrophobic surface area H H H H H C C C C H H H H H c. Lowest solubility. All of it's surface is non-polar. H O Indicates hydrophobic surface H H H C C H H a. Highest solubility. It has about the same size non-polar surface as c, but it has more sites for hydrogen-bonding with water. H H O H O H H O O H H H H H H C H b. Intermediate solubility. It has fewer hydrogen bonding sites than a. C H O C H H H O 1. The following mechanism has been proposed: k1 Step 1 O3 O2 + O k2 Step 2 O + O3 2 O2 a) Write a balanced equation for the overall reaction. b) Write the predicted rate law for the overall reaction if k2 is much larger than k1. c) Identify any reaction intermediates. a) Add the equations: O3 + O + O3 O2 + O + 2 O2 Combine terms and cancel out what is the same on both sides. the result is: 2 O3 3 O2 b) Rate = k1[O3]1 c) The only intermediate (produced in an early step and used in a later step) is the oxygen atom, O. 2. Given these data for the reaction of Fe3+ with I- : 3+ - 2+ 2 Fe (aq) + 2 I (aq) 2 Fe (aq) + I2 (aq) Exp# 1 2 3 [Fe3+] (M) 0.0400 0.0800 0.0400 - [I ] (M) 0.0300 0.0300 0.0600 Initial rate (M/s) -4 8.10 x 10 1.62 x 10-3 3.24 x 10-3 Be sure to explain the logic for your answers. a) Write a general rate law for the reaction. b) Use the initial rate data to write the specific rate law consistent with the data. Make sure you solve to obtain a numerical value for k. c) Write a chemical rxn. for the reactant side of the rate limiting step in the rxn. mechanism. d) What is the initial rate of the rxn. with 0.090 M Fe3+ and 0.50 M I-? e) If I told you that the value of k that you obtained is relatively large, what height hill would you draw for this reaction on a reaction progress diagram (vertical axis = G°). a) Rate = k [Fe3+]m[I-]n b) Compare trials 1 and 2: Rate2 = k [Fe3+]m[I-]n Rate1 = k [Fe3+]m[I-]n k/k cancels as does [0.0300]n/[0.0300]n, so Compare trials 1 and 3: Specific rate law is 1.62 x 10-3 = [0.0800]m 8.10 x 10-4 = [0.0400]m Rate3 = k [Fe3+]m[I-]n Rate1 = k [Fe3+]m[I-]n k/k cancels as does [0.0400]m/[0.0400]m, so k = Rate [Fe3+]m[I-]n 1.62 x 10-3 = k [0.0800]m[0.0300]n 8.10 x 10-4 = k [0.0400]m[0.0300]n or 2 = 2m , m = 1 3.24 x 10-3 = k [0.0400]m[0.0600]n 8.10 x 10-4 = k [0.0400]m[0.0300]n 3.24 x 10-3 = [0.0600]n 8.10 x 10-4 = [0.0300]n filling in data from trial 1 k = or 4 = 2n , n = 2 8.10 x 10-4 [0.0400]1[0.0300]2 = 22.5 Rate = 22.5[Fe3+]1[I-]2 c) Fe3+ + 2I- d) Rate = 22.5[0.090]1[0.50]2 = 0.50625 or 0.51 M/s e) If k is relatively large, than the rate is relatively fast and the activation energy hill would be relatively small. 3. Explain how/why changes in temperature result in a change in rate constant, k. Include an appropriate graph. Molecules move with a distribution of speeds. When temperaturechanges, the distribution changes. For higher temperatures, the average speed and the top speed of a population of molecules would increase. The highest speeds are particularly imprtant because only they have suffiennt energy to reach the transition state in a collision (in other words reach the top of the energy barrier, the top of Ea hill). Because a larger fraction of molecules is moving at a high enough speed to Boltzmann Distributions have their collisions reach the transition state, a larger fraction of collisions will be productive, and the rate of the reaction will increase. For lower temperatures, there is a decrease in the fraction of molecules that can reach the transition state. colder temperature frequency warmer temperature speeds that could overcome Ea barrier. 5. Write equilibrium constant expressions for the following reactions: a) 2 H2 (g) + 2 NO (g) 2 H2O (g) + N2 (g) b) Fe (s) + CO2 (g) FeO (s) + CO (g) a) Keq = speed [H 2 O]2 [N 2 ] [H 2 ]2 [NO]2 b Keq = [CO] [CO 2 ] 6. Shown below is a balanced equation for the decomposition of H2S to form H2 and S2. 2 H2S (g) 2 H2 (g) + S2 (g) a) Write an equilibrium constant expression for the reaction [H 2 ] 2 [S2 ] . Keq = [H 2 S]2 b) Given the equilibrium concentrations: [H2S] = 0.1007 M, [H2] = 0.0219 M, and [S2] = 3.30 × 10-3 M, calculate the numerical value of Keq. Keq = [0.0219]2 [3.30 x 10 -3 ] = 1.56 x 10-4 2 [0.1007] c) Assume the equilibrium is perturbed. When equilibrium is reestablished, the following concentrations are observed: [H2] = 0.00287 M and [S2] = 0.171 M. Calculate [H2S] under these new conditions. Rearrange the equation in (a) [H2S]2 = [H 2 ] 2 [S 2 ] [0.00287] 2 [0.171] = = 9.0289 x 10-3 M2 K eq 1.56 x 10 -4 [H2S] = 9.0289 x10 −3 = 0.0950 M d) What can you say about the forward and reverse reaction rates when the system is at equilibrium? At equilibrium, the rate of the forward reaction equals the rate of the reverse reaction. e) What can you say about the forward and reverse rate constants, whether the system is at equilibrium or not? kf kr = Keq 7. a) Qualitatively, for the reaction shown #6, would the ∆G° value be positive or negative? Explain your logic. ∆G would be positive, because Keq < 1. b) Assuming I told you the reaction in #6, was relatively fast. Draw a reaction coordinate diagram that describes that system. Then draw another showing how the system would be changed in the presence of a catalyst. Be sure to clearly label all important quantities. (The Keq determined in #6 was less than 1, so the reaction is reactant favored. transition state (top of hill) transition state (top of hill) Eact uncatalyzed 2H2 + S2 A relatively fast reaction means a relatively small hill. (Actually write relatively small hill next to drawing.) 2H2 + S2 Eact catalyzed Go Change in G is positive 2 H2S uncatalyzed reaction (small hill) Reaction Progress Go Change in G is positive 2 H2 S catalyzed reaction (smaller hill) Reaction Progress 8. What is the definition of a Brønsted-Lowry acid? A Brønsted-Lowry acid is a substance that can donate an H+. 9. Identify the acid and base on the reactant side of the equation shown below. Predict the products of the reaction and indicate the conjugate of each reactant. NH3 + HBr NH3 + base HBr acid NH4+ + conjugate acid Br conjugate base 10.(a) Write a balanced chemical reaction for the dissociation of acetic acid (CH3COOH) in water, and then (b) write the Ka expression for that rxn. Acetic acid is a relatively weak acid. (c) What could you say about the strength of its conjugate base? Formic acid is a slightly stronger acid than acetic acid. (d) Would formic acid dissociate to a greater or lesser extent in water compared to acetic acid? (e) Which acid would have the larger pKa? a) CH3COOH + H2O CH3OO- + H3O+ b) Ka = [CH 3 COO - ] [H 3 O + ] CH 3 COOH c) A relatively strong conjugate base d) Formic acid would dissociate to a greater extent e) Acetic acid would have the larger pKa 11.(a) Calculate the [H3O+], [OH-], and pH of a 7.00 x 10-3 M solution of HNO3. (b) Is this solution acidic, basic or neutral? (c) Would the pH be the same for a 7.00 x 10-3 M solution of acetic acid? Explain. + -3 a) HNO3 is a strong acid and completely dissociates, so [H3O ] = 7.00 x 10 M. pH = -log (7.00 x 10-3) = 2.155 −14 1x10 Kw -12 Kw = [H3O ] [OH ] Rearrange to get [OH ] = = M −3 = 1.43 x 10 + 7.00 x10 [H 3 O ] b) acidic (You can tell because pH < 7, and/or because [H3O+] < 1 x 10-7 M.) + - - c) The pH would be higher for a solution of acetic acid, because it is a weak acid and doesn’t dissociate as much as a strong acid like HNO3. 12. How does a buffer function to keep the pH of a solution relatively constant when strong acid or base is added? Make sure your answer includes equations for chemical reactions. A buffer is composed of reasonably equal amounts of a weak acid (HA) and its conjugate base (A-). The weak acid neutralizes added strong base (for example OH-). HA + OH- A- + H2O The conjugate base, A-, neutralizes added strong acid (for example H3O+). A- + H3O+ HA + H2O 13. What weak acid could be used to make a buffer that was effective around pH 4.25? You must explain your answer thoroughly to receive full credit. An effective buffer must have reasonable quantities of both weak acid (to react with added base) and conjugate base (to react with added acid) components. Ideally [HA] = [A-]. From the Henderson-Hasselbalch expression, when [HA] = [A ], then pH = pKa. Because the desired pH of the buffer is 4.25, we need a weak acid with a pKa near 4.25. Looking at the accompanying list of pKa for various acids, we can see that benzoic acid, with a pKa of 4.19 would be suitable. (The second dissociation of oxalic acid, with a pKa2 of 4.19 would also work.) 14. Write a balanced chemical reaction for the dissociation of water, and then write the Kw expression H2O + H2O H3O+ + OH- for that rxn. Kw = [H3O+][OH-] 15. Why (chemically) are you inhaling? A person inhales to take up oxygen gas, O2. Why (chemically) are you exhaling? A person exhales to remove carbon dioxide, CO2. Which of these would you expect to have the most direct affect on acidosis. Explain using equations. Carbon dioxide, CO2, should have the most direct affect on acidosis, because CO2 reacts with water to form carbonic acid (a weak acid): H2O + CO2 H2CO3 Carbonic acid dissociates in water to form H3O+, which is the direct cause of acidosis: H2CO3 + H2O HCO3- + H3O+ 16. Identify four functions that must be achieved for living things to stay living. Briefly describe each function and indicate how it contributes to staying alive. In class we identified 6: 1. Make catalysts 2. Extract energy from environment 3. Extract material from the environment. 4. Store and express genetic information 5. Maintain boundries (membranes) 6. Other specialty functions See your notes for details. 17. Draw a generalized structure for an amino acid (use may use “R” for the side chain”). H H amino group O N H C C carboxyl group H O R R represents the side chain + H H N C H R O C H O - Predominant structure at neutral pH 18. Show how two amino acids join to form a peptide bond. 19. Why would it be very appropriate for the amino acid, aspartic acid, to lie on the outer surface of a cytoplasmic protein? A cytoplasmic protein is surrounded by water which is polar. Aspartic acid’s side chain is polar and negatively charged at neutral pH. It is hydrophilic. 20. What are the levels of structure of proteins? What kinds of forces/bonds maintain this structure? Primary (1o) structure of proteins is the amino acid sequence. It is maintained by covalent bonds called peptide bonds. Secondary (2o) structure is a regular repeating structure due to folding of the polypeptide chain. The main types are alpha-helix and beta sheet (either parallel or anti-parallel). Secondary structure is maintained by hydrogen bonds formed between a hydrogen (donor) attached to the nitrogen in the backbone of the chain and the non-bonding pair on the carbonyl oxygen (C=O) in the backbone. Tertiary (3o) structure refers to the location of each atom of the protein relative to every other atom in three dimensional space. This structure is maintained by hydrophobic interactions, hydrogen bonding, covalent (disulfide, -S-S-) bonds, ionic bonds and London forces. Quaternary (4o) structure exists only for proteins that have more than one chain or subunit. It describes the way the subunits are arranged and bind to each other. It is maintained by the same forces as tertiary structure. 21. Given an organic molecule, be able to circle the chiral carbons. Example with chiral carbons circled: Br H H H H C C C C Cl Br H O